 Evolution
Evolution
 Intelligent Design
Intelligent Design
Genetic Similarities Between Fins and Limbs — Evidence for Evolution, Maybe, but Not for Darwinism
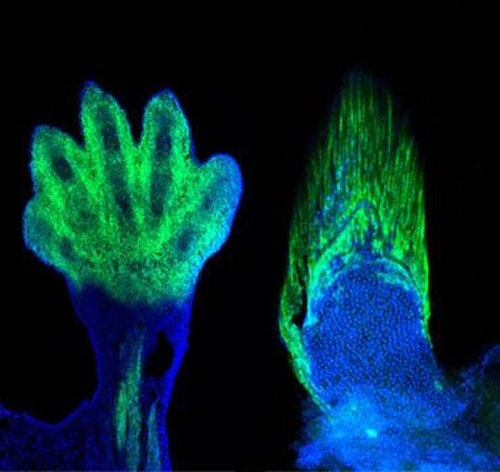
In a recent New York Times article, Carl Zimmer described new research from Neil Shubin’s lab at the University of Chicago on the developmental genetics of fish fins. He rightly points out that the work reveals (or more properly confirms, as previous evo-devo work points in very much the same direction) that very deep similarities exist between the development of fins and limbs. This is not surprising. It has been acknowledged since the time of Richard Owen, 160 years ago, that the lateral appendages of all vertebrates (fins of fish and limbs of tetrapods) are homologous. I expect that many more homologous gene expression patterns, gene circuits, etc., in the development of vertebrate lateral appendages will be elucidated in coming decades.
The experiments concerned two genes, Hoxa-13 and Hoxd-13, which researchers have known for some time are expressed in developing limbs. In the 1990s, French researchers shut down these two genes in mouse embryos and showed that while the mice developed normal long bones in their legs, “their wrist and ankle bones failed to appear, and they did not grow any digits.” This implies that these genes in “normal tetrapods” direct cells in the extremity to make endochondral bone (the ordinary boney material that makes up our skeleton and that of all tetrapods). In fish fins, the bony material that makes up the rays is somewhat different, called “dermal bone.” It was a long-standing puzzle to know how the switch was made from fish to tetrapod.
But when researchers in Shubin’s lab knocked out the same two genes — Hoxa-13 and Hoxd-13 — in fish, the fish developed without fin rays. This implies that the two genes in tetrapods tell certain cells to make proper endochondral bone, and tell cells in fish to make dermal bone. In a second experiment, another researcher in Shubin’s lab showed that it is the same group of cells in the same distal region of the developing fin and limb that express Hoxa-13 and Hoxd-13 to make dermal fin ray bone in fish or endochondral bone in tetrapods. Obviously the developmental genetic gap between fin and limb is diminished by these results and the deep homology of all vertebrate appendages is further supported.
I think Zimmer’s conclusion is fundamentally right, that is, as he puts it: “The unexpected discovery will help researchers understand how our own ancestors left the water, transforming fins into limbs that they could use to move around on land.” However, I think he has omitted some of the important and obvious challenges to the Darwinian framework implicit in this work. On any consideration, these advances (and many other similar in the evo-devo area) provide no support for the Darwinian causal framework — a long sequence of small adaptive changes unconstrained by any factors other than immediate adaptive utility — as sufficient to propel the evolution of the limb from fins.
This evidence provides further support for the notion that the genotypic distance between fin and limb may be far smaller than previously envisaged. It is indeed intriguing that, as in other areas of evo-devo, the less the genetic distance across an evolutionary transition, the more the evidence for evolution grows. But ironically, at the same time, the less room there is for Darwinism as a creative agency. Its as if the hand was already in the developmental genetics of the fin, so to speak, awaiting evolutionary expression, perhaps generated by a relatively small genetic trigger. At the limit one might envisage one base change initiating a series of deterministic developmental changes converting fin to limb — or more realistically, an upstream mutational change in a regulatory circuit switching the downstream expression to generate a limb instead of a fin and endochondral bone instead of dermal.
On such a scenario, there is absolutely nothing left for the agency of cumulative selection to do. Evolution would be completely constrained by the deep homologies underlying all vertebrate lateral appendages and is just an unfolding of alternative phenotypes already inherent in these shared commonalities. Moreover, on the same scenario, the evolutionary transition could have occurred quite suddenly as the fossil evidence in fact suggests, there being no intermediates known between fin rays and digits in any putative intermediate form.
That the transition might have been quite sudden, without any or only marginal selective surveillance, is born out by other considerations. For example, the similarity of the basic hind and fore limb (foot and hand) design cannot be ascribed to Darwinian selection because in no known fish or tetrapod is the morphology of the fore and hind appendage identical. This is not surprising, as they serve different functions in every known species (exemplified by the human hand and foot). There is therefore no way that natural selection could have assembled an identical pattern in both appendages bit-by-bit. In fact the two appendages represent a case of serial homology and it is universally assumed that there was a re-deployment of the same pattern from hind to fore limb, or vice versa.
But this means that in the case of at least one of the appendages, a design was imposed on the appendage without selective surveillance. This redeployment was in essence by definition non-adaptive, and may well have occurred per saltum. And the same applies to the digits — all have exactly the same design (as is obvious in the human fingers and toes). To explain this similarity in terms of classic Darwinism, envisaging that natural selection put together one digit to serve some adaptive end and then a second that “just happened” to have arrived at the same design, is too bizarre to contemplate. The digits are another case of serial homology. Again common sense dictates that the same design must have been re-deployed on all digits and it is reasonable to suppose that such a macromutational event might have occurred per saltum.
So yes, the increasing homology at the developmental genetic level is closing the gap. But the absence of any intermediates in the fossil record between fin rays and digits raises the serious possibility of per saltum changes. The very switch from cells making dermal bone to making endochondral bone, the event that is the focus of these studies, is again consistent with a saltational genetic switch and a non-Darwinian evolutionary model.
In short, my assessment is this: There never were any transitional forms making both dermal bone and endochondral bone. Organisms made one or the other.There never were any transitional forms with fin rays and digits. And I predict that no matter how extensively the fossil record is searched, the phenotypic gap between fins and limbs will remain even as the genetic gap continues to diminish.
Image: Mouse limb and fin ray, by Shubin Laboratory, University of Chicago Medical Center, via EurekAlert.
