 Evolution
Evolution
 Intelligent Design
Intelligent Design
 News Media
News Media
Researchers Proclaim: Instant Animals by Chance
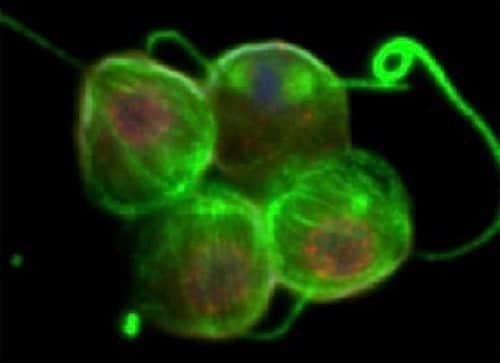
An old preacher wrote in his sermon notes, “Point weak; pound pulpit harder.” That seems to happen, too, whenever a major new success for evolution is announced. A recent headline from the University of Oregon proclaimed, “A mutation, a protein combo, and life went multicellular.” Sarah Kaplan at the Washington Post rose to the pulpit and began with a stirring invocation. “Startling new finding: 600 million years ago, a biological mishap changed everything.”
How? Well, in the high-stakes game of evolution, mutations in DNA that get passed on can be like bad photocopies, but…
But if the flaw is wrong in exactly the right way, the incredible can happen: disease resistance, sharper eyesight, swifter feet, big brains, better beaks for Darwin’s finches. [Emphasis added.]
Before we can catch our breath, Kaplan pounds the pulpit harder:
In a paper published in the open-access journal eLife this week, researchers say they have pinpointed what may well be one of evolution’s greatest copy mess-ups yet: the mutation that allowed our ancient protozoa predecessors to evolve into complex, multi-cellular organisms….
Incredibly, in the world of evolutionary biology, all it took was one tiny tweak, one gene, and complex life as we know it was born.
Kaplan has not even reached the climax of her rhetoric, but before getting swept away by the performance, let’s pause to look at the evidence. It comes from a paper in eLife by principal author Kenneth Prehoda at the University of Oregon who, with eight others, reported on the “Evolution of an ancient protein function involved in organized multicellularity in animals.”
Basically, they claim that a single mutation “repurposed” an enzyme that made multicellularity possible. A common guanylate kinase enzyme (gk), used by all living things to regulate the supply of nucleotides for the genetic code, underwent a mutation that enabled it to learn a new function. The new GKPID enzyme, found primarily in animals and choanoflagellates, is important for cell adhesion and spindle orientation. The mutation gave it a new shape that enabled it to bind to a different ligand. Sometime later, GKPID found a new partner in Pins, a protein on the inner membrane that (with some helper enzymes) connects to both the spindle microtubule and the complex that receives signals from neighboring cells. Astrobiology Magazine explains why this appears significant:
In cells from a broad range of animal species, the spindle is rotated relative to surrounding cells by a protein scaffold known as the guanylate kinase protein interaction domain (GK-PID). It acts as a kind of molecular carabiner by binding to two different partner molecules: an ‘anchor’ protein on the inside of the cell membrane that indicates the position of adjacent cells and a motor protein that pulls on mitotic spindle filaments. Once hooked together by GK-PID, the motors pull the chromosomes toward the anchors, orienting new daughter cells in line with neighboring cells.
Prehoda gives the gist of the idea himself in a video clip:
It’s a neat story; a random mutation at a critical juncture in the history of life opens up a world of possibilities for cells to work together. It’s just what Darwin dreamed of: an unguided process, co-option, innovation, at the right place and the right time to create endless forms most beautiful. No wonder this story reverberated around the world in hyped headlines like Kaplan’s. Pound the pulpit harder!
In the wild world of pre-complex life, this development was orders of magnitude better than Twitter for getting organisms organized. Every example of cells collaborating that has arisen since — from the trilobites of 500 million years ago to the dinosaurs, woolly mammoths and you — probably relied on it or some other similar mutation.
We’ll have to add this one to the explanations for the Cambrian explosion. Mutation — trilobites!
You can’t blame Kaplan and other reporters for taking this supposition and running up the whole evolutionary tree with it. In Astrobiology Magazine’s coverage, co-author Joe Thornton said, “That one ancient mutation yielded a wholly new molecular function, which helped set the stage for multicellular animals to eventually evolve.” Indeed, Prehoda told the Oregon Register-Guard newspaper:
From a microbiology standpoint, Prehoda said, there’s no argument about evolution. “You can make evolution happen on a rapid time scale in the lab,” he said. “We’ve witnessed evolution. Evolution is just a fact, hands down.”
Prehoda said this, incidentally, in response to “the ire of anti-evolutionists” when the story went viral. Reporter Diane Dietz says in her article, “University of Oregon researcher’s paper on evolution stirs debate; he says the transformation of single-celled organisms to multi-celled creatures occurred more easily than many scientists believed.” She continues:
A paper on evolutionary biology he and co-authors published this month on eLifeSciences, an electronic scholarly journal, was ground-breaking enough that scientists nationally took notice — and so provocative that it became clickbait for opponents of evolutionary theory.
Those anti-evolutionists. All they’ve got is religion.
By contrast, the so-called intelligent design theory put forth by believers who say a divine entity created humans is based on the idea that organisms are so complex that they couldn’t arise from the random, step-by-step process of evolution. As a result, Prehoda now finds his email box stuffed with missives from unhappy anti-evolutionists.
The writers’ general message is: “You say we come from cells and monkeys, but we come from God,” Prehoda said.
Obviously, that is not how ID advocates argue. Prehoda’s story is so beset by scientific and logical flaws it doesn’t need divine assistance to point it out. Ann Gauger gave a calm, scholarly critique of a similar claim about the origin of multicellularity last year, without any appeals to God, religion, or personal belief. Following her example:
-
The claim relies on circumstantial evidence.
-
It’s a little hard to do “molecular time travel” when working with living cells, without presupposing evolutionary common ancestry.
-
If the guanylate kinase was co-opted to become GKPID, what about all the other things it connects to? Was everything co-opted from something else, including the Pins complex on the membrane, and the microtubules in the spindle complex? You can’t push co-option too far, or else you end up borrowing from nothing.
-
It’s nice that cells with GKPID can orient their spindles to neighboring cells, but that’s a far cry from multicellularity. If one cell gets the mutation, it still doesn’t have any goal to line up with its neighbor. Nothing interesting will happen (unless one presupposes that “evolution” will latch onto this new capability). Indeed, if all the choanoflagellates get the mutation, the best they could do is blindly point to neighboring cells in an unguided, chaotic manner.
-
The signal from the neighboring cell has to be interpreted. Nothing interesting will happen if the mutant hollers, “I’ve got my carabiner ready!” and the neighbor is deaf, or responds, “No comprendo.”
-
Ann Gauger’s critique of the previous such claim bears repeating: much, much more is involved. One of the “simplest” colonies of all, Volvox, has sexual reproduction, alternation of generations, inversion, digestive enzymes, gene regulation, specialized roles, and more.
-
The authors use loaded words like ancient and primitive copiously. These terms presuppose what they need to prove about evolution. There’s nothing primitive about a choanoflagellate, with its genetic code, ribosomes, flagellum and all the complexities of a living cell.
-
Even if a guanylate kinase differs from GKPID by one mutation, they are both functional within complex systems and regulatory networks. Many other proteins are structurally similar but have different functions. Similarity does not prove ancestry.
-
If GKPID appeared by mutation, how did the rest of the cell know what to do with it? There has to be genetic coding and gene regulation. Unless the new function is encoded in harmony with the systems that regulate it, it will be treated as a defect and eliminated. To think it will immediately be useful smacks of Lamarckism.
-
The authors admit a “long time” gap between the mutation and the ability to link up to the Pins complex and the spindle orientation complex. Was this a “latent capacity” sitting around waiting to be utilized? “We agree that this is puzzling,” they admit. “Because whatever we say here would be very speculative, we did not go into much detail on this point.” Even more puzzling, the GKPID does not bind to the Pins complex in the same organism, but only to one from a fruit fly!
In a moment of epistemic modesty, the authors admit that their supposition doesn’t really amount to much. It’s a case of glittering generalities at best, sweetened with high hopes.
Our analyses do not establish a complete history of the spindle orientation complex. Many key steps remain to be reconstructed, including how and when the interaction between GKPID and KHC-73 evolved, the mechanisms by which Pins’ acquired its linker and GoLoco sequences, and the relationship of these components to other molecular complexes and pathways involved in animal spindle orientation. Despite these knowledge gaps, our observations establish a broad overview of the history of the GKPID complex, provide a detailed mechanistic reconstruction of a key event, and point to the importance of reusing molecules — and specific surfaces within them — for fortuitous new purposes that have the potential to become biologically essential.
Unusual for a journal paper, this one includes the dialogue between the reviewers and the authors. The criticisms and responses are well worth reading. Despite the editors’ interest in publishing this paper, they were clearly concerned about the authors’ tendency to overstate their case.
Our major critique is that the broader interpretation is overstated in terms of the centrality of KHC73-DLG-PINS to spindle orientation in all animals and multicellularity in general, and in terms of external orientation being a unique novelty of animals. We also think that some clarification / caveats are needed regarding the experiments on positioning in Choanoflagellates. Finally, we think the manuscript would benefit from more discussion of some puzzling aspects of the co-evolution of GKPID and PINS (or lack thereof).
The editors charged “overstated” multiple times. Embarrassed, the authors confessed and repented somewhat:
We have modified the text in numerous ways to be more cautious on this point and to base our claims more solidly on what is known in the literature….
More generally, we have gone through the text and have changed our wording to dispel the impression that GKPID complex is the sole driver of spindle orientation in all animals and all cell types and to avoid the implication that the evolution of the GKPID complex explains all instances of spindle orientation in all animals.
Unfortunately, this confession didn’t make it into Kaplan’s sermon or into Prehoda’s bombast, “We’ve witnessed evolution. Evolution is just a fact, hands down.” Overstated? That’s an understatement.
Image credit: Choanoflagellate colony, by Ken Prehoda via University of Oregon.
