 Intelligent Design
Intelligent Design
 Medicine
Medicine
Pumping for Life: What the Sodium-Potassium Pump Accomplishes
Editor’s note: Physicians have a special place among the thinkers who have elaborated the argument for intelligent design. Perhaps that’s because, more than evolutionary biologists, they are familiar with the challenges of maintaining a functioning complex system, the human body. With that in mind, Evolution News & Views is delighted to present this series, “The Designed Body.” For the complete series, see here. Dr. Glicksman practices palliative medicine for a hospice organization.
 In this series we’ve seen what makes up the human cell and what it needs to do to survive, given the laws of nature. One of the main things the cell must do is control its chemical content and volume. If not combated by some sort of innovation, the natural forces of diffusion and osmosis have the potential to quickly bring about cell death. This is due to the fact that the chemical make-up of the fluid inside the cell is exactly the opposite of the fluid outside the cell, and the cell must let the chemicals it needs to live (like glucose) come in and the toxic ones it produces (like carbon dioxide) go out through the plasma membrane. In having a plasma membrane that is permeable to certain chemicals, but not to others (like most proteins), the cell must follow the rules — entailing that it is affected by the natural forces of diffusion and osmosis.
In this series we’ve seen what makes up the human cell and what it needs to do to survive, given the laws of nature. One of the main things the cell must do is control its chemical content and volume. If not combated by some sort of innovation, the natural forces of diffusion and osmosis have the potential to quickly bring about cell death. This is due to the fact that the chemical make-up of the fluid inside the cell is exactly the opposite of the fluid outside the cell, and the cell must let the chemicals it needs to live (like glucose) come in and the toxic ones it produces (like carbon dioxide) go out through the plasma membrane. In having a plasma membrane that is permeable to certain chemicals, but not to others (like most proteins), the cell must follow the rules — entailing that it is affected by the natural forces of diffusion and osmosis.
Diffusion has the potential to drastically alter the cell’s chemical content by naturally causing potassium leave the cell through its plasma membrane while causing sodium to enter. And while diffusion is trying to make potassium and sodium equalize within the fluid inside and outside the cell, osmosis has the potential to drastically alter the cell’s volume by naturally making water enter the cell at the same time because its large amount of protein can’t cross the plasma membrane. Together, the effects of diffusion and osmosis can give the cell a one-two punch, quickly resulting in death. What kind of mechanism could possibly do the job of controlling not only the cell’s chemical content but its volume too?
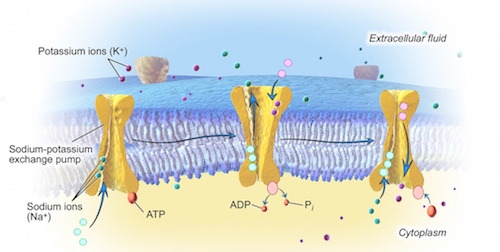
Consider what you would have to do if you were sitting in a boat that constantly had water leaking into it. Of course, you would have to constantly remove that water, otherwise the boat will sink. But, what if your only option is to keep the boat in the water and you can’t be there to do the work of bailing all the time? Could you place a machine in the boat to do the work for you? That is, a pump. This is precisely the type of micro-machine the cell uses to take control of its chemical content and volume. In fact, the cell has a few million of these sodium-potassium pumps within its plasma membrane.
The sodium-potassium pump acts by pushing sodium out of the cell and pulling potassium back in. Even though the laws of nature make sodium go into, and potassium go out of, the cell as they diffuse down their respective concentration gradients, the millions of sodium-potassium pumps in the plasma membrane immediately reverse most of this movement. In fact for every three ions of sodium that are pumped out of the cell, two ions of potassium are pumped back in.
This is how the cell reverses the natural tendency for the fluid inside and outside to have equal concentrations of sodium and potassium. In so doing, it maintains its chemical content. However, the action of the sodium-potassium pump not only preserves the cell’s chemical content, it also controls its volume by preventing water from entering as. Here is how.
Remember, as chemicals like sodium and potassium move across the permeable plasma membrane and diffuse down their concentration gradient, water rushes into the cell due to the large amount of impermeable protein pulling it in by osmosis. In other words, in biology, a solute exerts an osmotic pull on water across a membrane based on its inability to leave that solution. Again, since protein can’t leave the fluid in the cell, because it can’t go through the plasma membrane, it’s able to apply an osmotic pull on the water outside the cell and bring it inside. Since sodium and potassium freely pass across the plasma membrane, they should not be able to apply an osmotic pull on water in either direction. Or can they?
With the sodium-potassium pumps in the plasma membrane of the cell pushing most of the sodium back out of the cell and bringing most of the potassium back in, although they are still permeable, they now effectively act as if they were impermeable. By forcing sodium and potassium to stay where they are, the sodium-potassium pumps give them the power to move water toward them by osmosis. As noted above, in biology, a solute exerts an osmotic pull on water across a membrane based on its inability to leave that solution. With the sodium-potassium pumps forcing sodium to stay outside the cell and keeping potassium inside, they have effectively made them unable to leave their solution. In doing so, the sodium-potassium pumps have also made sodium and potassium osmotically active chemicals, just like the protein inside the cell.
This means that, not only does protein have a tendency to pull water into the cell from the fluid outside, but so does potassium as well. In addition, since the sodium-potassium pumps push sodium out of the cell, not letting it stay on the other side of the plasma membrane, it also enables sodium to pull water from inside the cell back outside. The osmotic pull of sodium from outside the cell is in the opposite direction to the osmotic pull exerted by the protein and potassium inside it. In fact, the cell is very sensitive to water movement in either direction across its plasma membrane, which directly affects its volume. To take control of its volume the cell always tries to make sure that the osmotic pull of water from the fluid outside the cell evenly matches the pull to bring water back in. It does this by making certain that the concentration of total chemical particles in the cytosol is the same as in the fluid outside the cell. When this is achieved, the fluids are said to be isotonic.
This is what the sodium-potassium pump accomplishes. But there is a price to be paid by the body for thus battling the forces of nature. The job of the sodium-potassium pump is like having to walk against a strong driving wind. The effort, needed for survival, requires tremendous energy. At rest, between one-quarter to one-half of the total energy needs of the body are taken up by the millions of sodium-potassium pumps in each of its trillions of cells. This goes to show that real numbers have real consequences. If the cell doesn’t have enough energy to power its millions of sodium-potassium pumps, it is as good as dead. But where does the cell get the energy it needs? Before you can begin to understand the answer to this question, you must first learn about enzymes and how they work in the body. We’ll look at them next time.
Image by Blausen.com staff. “Blausen gallery 2014”. Wikiversity Journal of Medicine. DOI:10.15347/wjm/2014.010. ISSN 20018762. (Own work) [CC BY 3.0 (http://creativecommons.org/licenses/by/3.0)], via Wikimedia Commons.
