 Evolution
Evolution
Problem 6: Molecular Biology Has Failed to Yield a Grand “Tree of Life”
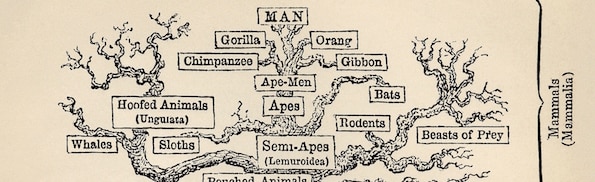
Editor’s note: This is Part 6 of a 10-part series based upon Casey Luskin’s chapter, “The Top Ten Scientific Problems with Biological and Chemical Evolution,” in the volume More than Myth, edited by Paul Brown and Robert Stackpole (Chartwell Press, 2014). The full chapter can be found online here. Other individual installments can be found here: Problem 1, Problem 2, Problem 4, Problem 5, Problem 6, Problem 7, Problem 8, Problem 9, Problem 10.
When fossils failed to demonstrate that animals evolved from a common ancestor, evolutionary scientists turned to another type of evidence — DNA sequence data — to demonstrate a tree of life. In the 1960s, around the time the genetic code was first understood, biochemists Émile Zuckerkandl and Linus Pauling hypothesized that if DNA sequences could be used to produce evolutionary trees — trees that matched those based upon morphological or anatomical characteristics — this would furnish “the best available single proof of the reality of macro-evolution.”99 Thus began a decades-long effort to sequence the genes of many organisms and construct “molecular” based evolutionary (“phylogenetic”) trees. The ultimate goal has been to construct a grand “tree of life,” showing how all living organisms are related through universal common ancestry.
The Main Assumption
The basic logic behind building molecular trees is relatively simple. First, investigators choose a gene, or a suite of genes, found across multiple organisms. Next, those genes are analyzed to determine their nucleotide sequences, so the gene sequences of various organisms can then be compared. Finally, an evolutionary tree is constructed based upon the principle that the more similar the nucleotide sequence, the more closely related the species. A paper in the journal Biological Theory puts it this way:
[M]olecular systematics is (largely) based on the assumption, first clearly articulated by Zuckerkandl and Pauling (1962), that degree of overall similarity reflects degree of relatedness.100
This assumption is essentially an articulation of a major feature of the theory – the idea of universal common ancestry. Nonetheless, it’s important to realize that it is a mere assumption to claim that genetic similarities between different species necessarily result from common ancestry.
Operating strictly within a Darwinian paradigm, these assumptions flow naturally. As the aforementioned Biological Theory paper explains, the main assumption underlying molecular trees “derives from interpreting molecular similarity (or dissimilarity) between taxa in the context of a Darwinian model of continual and gradual change.”101 So the theory is assumed to be true to construct a tree. But also, if Darwinian evolution is true, construction of trees using different sequences should reveal a reasonably consistent pattern across different genes or sequences.
This makes it all the more significant that efforts to build a grand “tree of life” using DNA or other biological sequence data have not conformed to expectations. The basic problem is that one gene gives one version of the tree of life, while another gene gives a highly different, and conflicting, version of the tree. For example, as we’ll discuss further below, the standard mammalian tree places humans more closely related to rodents than to elephants. But studies of a certain type of DNA called microRNA genes have suggested the opposite — that humans were closer to elephants than rodents. Such conflicts between gene-based trees are extremely common.
The genetic data is thus not painting a consistent picture of common ancestry, showing the assumptions behind tree-building commonly fail. This leads to justifiable questions about whether universal common ancestry is correct.
Conflicts in the Base of the Tree of Life
Problems first arose when molecular biologists sequenced genes from the three basic domains of life — bacteria, archaea, and eukarya — but those genes did not allow these basic groups of life to be resolved into a treelike pattern. In 2009, the journal New Scientist published a cover story titled, “Why Darwin was wrong about the tree of life” which explained these quandaries:
The problems began in the early 1990s when it became possible to sequence actual bacterial and archaeal genes rather than just RNA. Everybody expected these DNA sequences to confirm the RNA tree, and sometimes they did but, crucially, sometimes they did not. RNA, for example, might suggest that species A was more closely related to species B than species C, but a tree made from DNA would suggest the reverse.102
This sort of data led biochemist W. Ford Doolittle to explain that “Molecular phylogenists will have failed to find the ‘true tree,’ not because their methods are inadequate or because they have chosen the wrong genes, but because the history of life cannot properly be represented as a tree.”103 New Scientist put it this way: “For a long time the holy grail was to build a tree of life … But today the project lies in tatters, torn to pieces by an onslaught of negative evidence.”104
Many evolutionists sometimes reply that these problems arise only when studying microorganisms like bacteria — organisms which can swap genes through a process called “horizontal gene transfer,” thereby muddying the signal of evolutionary relationships. But this objection isn’t quite true, since the tree of life is challenged even among higher organisms where such gene-swapping is not prevalent. Carl Woese, a pioneer of evolutionary molecular systematics, explains:
Phylogenetic incongruities can be seen everywhere in the universal tree, from its root to the major branchings within and among the various taxa to the makeup of the primary groupings themselves.105
Likewise, the New Scientist article notes that “research suggests that the evolution of animals and plants isn’t exactly tree-like either.”106 The article explains what happened when microbiologist Michael Syvanen tried to create a tree showing evolutionary relationships using 2000 genes from a diverse group of animals:
He failed. The problem was that different genes told contradictory evolutionary stories. … the genes were sending mixed signals. … Roughly 50 per cent of its genes have one evolutionary history and 50 per cent another.107
The data were so difficult to resolve into a tree that Syvanen lamented, “We’ve just annihilated the tree of life.”108 Many other papers in the technical literature recognize similar problems.
Conflicts Between Higher Branches
A 2009 paper in Trends in Ecology and Evolution notes that, “A major challenge for incorporating such large amounts of data into inference of species trees is that conflicting genealogical histories often exist in different genes throughout the genome.”109 Similarly, a paper in Genome Research studied the DNA sequences in various animal groups and found that “different proteins generate different phylogenetic tree[s].”110 A June, 2012 article in Nature reported that short strands of RNA called microRNAs “are tearing apart traditional ideas about the animal family tree.” Dartmouth biologist Kevin Peterson who studies microRNAs lamented, “I’ve looked at thousands of microRNA genes, and I can’t find a single example that would support the traditional tree.” According to the article, microRNAs yielded “a radically different diagram for mammals: one that aligns humans more closely with elephants than with rodents.” Peterson put it bluntly: “The microRNAs are totally unambiguous … they give a totally different tree from what everyone else wants.”111
Conflicts Between Molecules and Morphology
Not all phylogenetic trees are constructed by comparing molecules like DNA from different species. Many trees are based upon comparing the form, structure, and body plan of different organisms — also called “morphology.” But conflicts between molecule-based trees and morphology-based trees are also common. A 2012 paper studying bat relationships made this clear, stating: “Incongruence between phylogenies derived from morphological versus molecular analyses, and between trees based on different subsets of molecular sequences has become pervasive as datasets have expanded rapidly in both characters and species.”112 This is hardly the only study to encounter conflicts between DNA-based trees and trees based upon anatomical or morphological characteristics. Textbooks often claim common descent is supported using the example of a tree of animals based upon the enzyme cytochrome c which matches the traditional evolutionary tree based upon morphology.113 However, textbooks rarely mention that the tree based upon a different enzyme, cytochrome b, sharply conflicts with the standard evolutionary tree. As one article in Trends in Ecology and Evolution observed:
[T]he mitochondrial cytochrome b gene implied . . . an absurd phylogeny of mammals, regardless of the method of tree construction. Cats and whales fell within primates, grouping with simians (monkeys and apes) and strepsirhines (lemurs, bush-babies and lorises) to the exclusion of tarsiers. Cytochrome b is probably the most commonly sequenced gene in vertebrates, making this surprising result even more disconcerting.114
Strikingly, a different article in Trends in Ecology and Evolution concluded, “the wealth of competing morphological, as well as molecular proposals [of] the prevailing phylogenies of the mammalian orders would reduce [the mammalian tree] to an unresolved bush, the only consistent [evolutionary relationship] probably being the grouping of elephants and sea cows.”115 Because of such conflicts, a major review article in Nature reported, “disparities between molecular and morphological trees” lead to “evolution wars” because “[e]volutionary trees constructed by studying biological molecules often don’t resemble those drawn up from morphology.”116
Finally, a study published in Science in 2005 tried to use genes to reconstruct the relationships of the animal phyla, but concluded that “[d]espite the amount of data and breadth of taxa analyzed, relationships among most [animal] phyla remained unresolved.” The following year, the same authors published a scientific paper titled, “Bushes in the Tree of Life,” which offered striking conclusions. The authors acknowledge that “a large fraction of single genes produce phylogenies of poor quality,” observing that one study “omitted 35% of single genes from their data matrix, because those genes produced phylogenies at odds with conventional wisdom.” The paper suggests that “certain critical parts of the [tree of life] may be difficult to resolve, regardless of the quantity of conventional data available.” The paper even contends that “[t]he recurring discovery of persistently unresolved clades (bushes) should force a re-evaluation of several widely held assumptions of molecular systematics.”117
Unfortunately, one assumption that these evolutionary biologists aren’t willing to re-evaluate is the assumption that universal common ancestry is correct. They appeal to a myriad of ad hoc arguments — horizontal gene transfer, long branch attraction, rapid evolution, different rates of evolution, coalescent theory, incomplete sampling, flawed methodology, and convergent evolution — to explain away inconvenient data which doesn’t fit the coveted treelike pattern. As a 2012 paper stated, “phylogenetic conflict is common, and frequently the norm rather than the exception.”118 At the end of the day, the dream that DNA sequence data would fit into a nice-neat tree of life has failed, and with it a key prediction of neo-Darwinian theory.
References:
[99.] Zuckerkandl and Pauling, “Evolutionary Divergence and Convergence in Proteins,” 101.
[100.] Jeffrey H. Schwartz, Bruno Maresca, “Do Molecular Clocks Run at All? A Critique of Molecular Systematics,” Biological Theory, 1(4):357-371, (2006).
[101.] Ibid.
[102.] Graham Lawton, “Why Darwin was wrong about the tree of life,” New Scientist (January 21, 2009).
[103.] W. Ford Doolittle, “Phylogenetic Classification and the Universal Tree,” Science, 284:2124-2128 (June 25, 1999).
[104.] Partly quoting Eric Bapteste, in Lawton, “Why Darwin was wrong about the tree of life” (internal quotations omitted).
[105.] Carl Woese “The Universal Ancestor,” Proceedings of the National Academy of Sciences USA, 95:6854-9859 (June, 1998) (emphasis added).
[106.] Graham Lawton, “Why Darwin was wrong about the tree of life,” New Scientist (January 21, 2009).
[107.] Partly quoting Michael Syvanen, in Lawton, “Why Darwin was wrong about the tree of life” (internal quotations omitted).
[108.] Michael Syvanen, quoted in Lawton, “Why Darwin was wrong about the tree of life.”
[109.] James H. Degnan and Noah A. Rosenberg, “Gene tree discordance, phylogenetic inference and the multispecies coalescent,” Trends in Ecology and Evolution, 24 (2009): 332-340.
[110.] Arcady R. Mushegian, James R. Garey, Jason Martin and Leo X. Liu, “Large-Scale Taxonomic Profiling of Eukaryotic Model Organisms: A Comparison of Orthologous Proteins Encoded by the Human, Fly, Nematode, and Yeast Genomes,” Genome Research, 8 (1998): 590-598.
[111.] Elie Dolgin, “Rewriting Evolution,” Nature, 486: 460-462 (June 28, 2012).
[112.] Liliana M. Dávalos, Andrea L. Cirranello, Jonathan H. Geisler, and Nancy B. Simmons, “Understanding phylogenetic incongruence: lessons from phyllostomid bats,” Biological Reviews of the Cambridge Philosophical Society, 87:991-1024 (2012).
[113.] For example, see BSCS Biology: A Molecular Approach (Glencoe/McGraw Hill, 2006), 227; Sylvia S. Mader, Jeffrey A. Isaacson, Kimberly G. Lyle-Ippolito, Andrew T. Storfer, Inquiry Into Life, 13th ed. (McGraw Hill, 2011), 550.
[114.] See Michael S. Y. Lee, “Molecular Phylogenies Become Functional,” Trends in Ecology and Evolution, 14: 177 (1999).
[115.]W. W. De Jong, “Molecules remodel the mammalian tree,” Trends in Ecology and Evolution, 13(7), pp. 270-274 (July 7, 1998).
[116.] Trisha Gura, “Bones, Molecules or Both?,” Nature, 406 (July 20, 2000): 230-233.
[117.] Antonis Rokas & Sean B. Carroll, “Bushes in the Tree of Life,” PLoS Biology, 4(11): 1899-1904 (Nov., 2006) (internal citations and figures omitted).
[118.] Liliana M. Dávalos, Andrea L. Cirranello, Jonathan H. Geisler, and Nancy B. Simmons, “Understanding phylogenetic incongruence: lessons from phyllostomid bats,” Biological Reviews of the Cambridge Philosophical Society, 87:991-1024 (2012).
Image by Ernst Haeckel [Public domain], via Wikimedia Commons.
