 Evolution
Evolution
 Intelligent Design
Intelligent Design
Thinking Differently About Biology
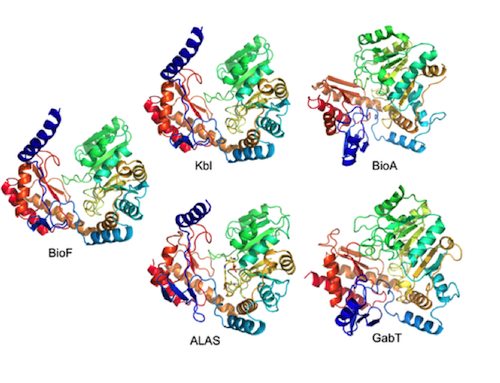
Five family members from the GabT-like protein family. The first three are very similar. These enzymes are considered by current standards to be homologous, that is, evolutionarily derived.
The five enzymes shown above are clearly related in structure, especially the three on the left. Yet none of the others can replace BioF2‘s function in the cell, even when mutated and made in large amounts. Why is that? Probably because each enzyme is a structural whole, whose sequence is made to work together as a whole. Substituting or changing little bits doesn’t work.
Here are the concluding paragraphs of our recent paper where we explain the problem and propose a new way of thinking about it:
Although there is as yet no satisfactory theory of biology to take the place of Darwinism, we believe the time has come for serious pursuit of such a theory. To quote one of our previous papers [45]:
The insights we gain from the critique of neo-Darwinism can and should inform the construction of a new theory to take its place. That is, in pinpointing the key problems with the old theory we are identifying crucial respects in which its replacement must differ from it. We ourselves have become convinced that intelligent causation is essential as a starting point for any successful theory of biological innovation. If this is so, what is needed now is an elaboration of the general principles by which living things have been designed.
To that end, one of our inferred principles of design is this [45]:
The substantial reworking of a homologous structure needed to give it a genuinely new function is more suggestive of reapplication of a concept than adjustment of a physical thing.
And another is this [45]:
The implementation of innovation is nearly the opposite of ordinary physical causation. It is the top-down arrangement of matter in such a way that the resulting bottom-up behavior of that matter serves the intended purpose of the innovator.
Taking these two ideas together, it may be that our prior attempts to convert Kbl2 to perform the function of BioF2 failed not because we made the wrong alterations but rather because it is misguided even to think of this as an exercise in alteration. Perhaps we should think of this more in the way we think about writing. Sentences that convey different ideas may have similar structures, but when we write a sentence we start with the idea, not the sentence structure. We never take a sentence that conveys some other idea and ask which letters can be changed to make it better suited for our present purpose. The fact that different ideas end up being conveyed with sentences of similar structure, then, has nothing to do with recycling of sentences and everything to do with the suitability of certain forms for certain functions.
Might this be the right perspective from which to view Kbl2 and BioF2? They use similar structures not because they are both adjusted versions of some older enzyme, but instead because the purposes they serve happen to call for similar structures. As we found in this work, it is not that Kbl has amino acid residues that are incompatible with the function of BioF2, but rather that Kbl2 is comprehensively suited to one function, while BioF2 is comprehensively suited to another. To us this change of perspective has the feel of a turn in the right direction. It does not in itself take us very far, perhaps, but having made the turn, forward progress may become much more likely.
Source:
Reeves MA, Gauger AK, Axe DD (2014) Enzyme families — Shared evolutionary history or shared design? A study of the GABA-aminotransferase family. BIO-Complexity 2014 (4):1-16. doi:10.5048/BIO-C.2014.4.
