 Evolution
Evolution
 Intelligent Design
Intelligent Design
The Flagellar Filament Cap: “One of the Most Dynamic Movements in Protein Structures”
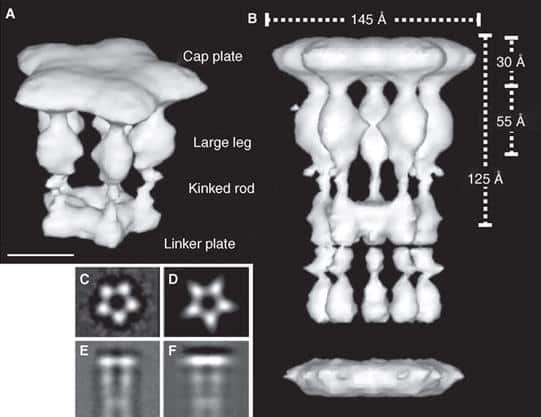
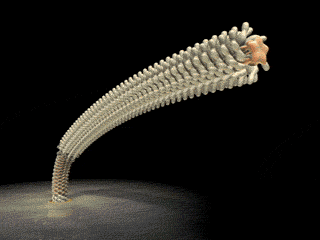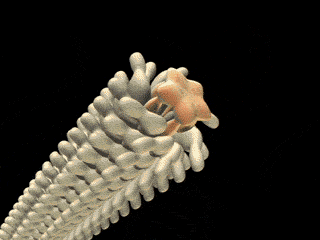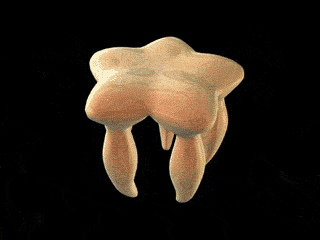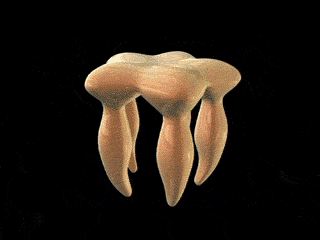
Imagine you are an archaeologist surveying the remains of a formerly technologically advanced civilization. You stumble across the device depicted in the figure above. With its pentagon-shaped plate and its carefully crafted leg-like extensions, it looks like it came from some sort of machine. Immediately, you intuitively infer that the device was designed intentionally, for a purpose. This isn’t the kind of device that is typically created by forces of nature such as wind and erosion.
You may be wondering what the device in the figure is, or what machine it forms a part of. The measurements indicate that, whatever it is, it is certainly extremely small. The figure, which was excerpted from Maki-Yonekura et al. (2003), in fact reveals a reconstructed three-dimensional image of the flagellar filament capping protein (also known as FliD). Here it is in context (credit for animations goes to Keiichi Namba et al. of the ERATO Protonic NanoMachine Project):
The activity of the capping protein of the bacterial flagellar filament — which serves as a rotary promoter for self-assembly of flagellin monomers — has been described as “one of the most dynamic movements in protein structures” (Yonekura et al., 2000).
 FliD is in fact critical to filament assembly. Without the presence of FliD, the flagellin monomers are lost (Kim et al., 1999). As one paper has explained, “A FliD-deficient mutant becomes non-motile because it lacks flagellar filaments and leaks flagellin monomer out into the medium” (Ikeda et al., 1996). Indeed, FliD is crucial for proper assembly of the flagellin proteins, as shown in the animation below. What is even more interesting still is that FliD presently has no known homologues in non-flagellar systems.
FliD is in fact critical to filament assembly. Without the presence of FliD, the flagellin monomers are lost (Kim et al., 1999). As one paper has explained, “A FliD-deficient mutant becomes non-motile because it lacks flagellar filaments and leaks flagellin monomer out into the medium” (Ikeda et al., 1996). Indeed, FliD is crucial for proper assembly of the flagellin proteins, as shown in the animation below. What is even more interesting still is that FliD presently has no known homologues in non-flagellar systems.
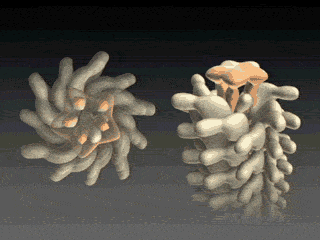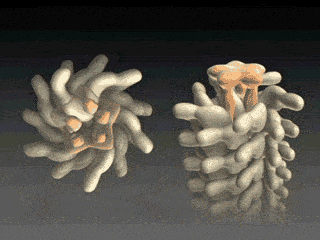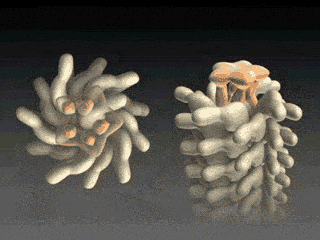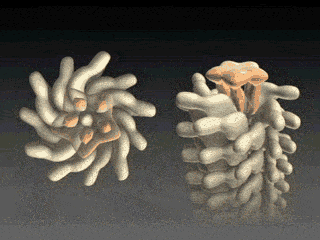
How, then does the capping protein work, and why is it so essential? The cap rests on top of the hollow flagellar filament, and possesses five leg domains that point downwards and insert into cavities at the distal tip of the nascent filament (Maki et al., 1998). The flagellin subunits (which are exported out the type III system and assemble at the distal-growing end of the filament) form a helical structure and possess 5.5 subunits per turn, and thus 5.5 subunits at the distal end of the growing filament. Since there are only 5 leg-like domains (leading to a symmetry mismatch), this means that there is always a small crevice or space at one point between the filament and cap plate. This indentation acts as a folding chamber for the newly-exported flagellin monomers which are added at this site (Yonekura et al., 2000). When a flagellin monomer has been incorporated into this space, the cap complex rotates and moves up, and a new indentation is created adjacent to the previous one that was just occupied (Minamino and Namba, 2004; Maki-Yonekuru et al., 2003). The rotation rate of the filament cap is approximately 600 rotations per minute, or 10 per second, and 50 flagellin monomers are added per second.
The hollow channel through which the flagellin monomers travel is extremely narrow — only two nanometers in diameter. Since flagellin subunits have a kink in the middle, they are too large to travel through the tube folded, and thus they pass through the tube unfolded. They are thus unable to fold properly by themselves, and it is the FliD cap that ultimately facilitates the folding, and proper positioning and assembly, of the flagellin monomers.
The filament cap thus needs to be of very specific structure in order to interact with, and direct the assembly of, the flagellin monomers that are exported. It is also crucial for flagellar assembly and one of the very last proteins to be added. How did a protein of such specific structure evolve and how long would it have taken? Surely, a filament capping protein (which serves no other purpose within the cell as far as we know) is of no use without the exporting of the flagellin monomers whose assembly it facilitates. But without the presence of such a capping protein, the flagellin monomers are of no value — since they are lost into the medium and do not assemble into a filament. Moreover, it is essential that only a single filament capping protein, but many specifically structured flagellin monomers (a filament is typically comprised of around 20,000 monomers), be exported. It is also essential that their respective export be in the correct order — FliD must be exported before the export of flagellin subunits. How long did it take for such a complex system to evolve?
Perhaps one could argue — as does Nick Matzke — that FliD and the filament proteins (FliC), in addition to the rod and hook proteins, evolved from an ancestral adhesive pilin secreted from the primitive type III secretion system. But such a hypothesis only pushes the need for explanation back a step — at some point, the elegant interaction between FliD and the flagellin monomers still needs to be accounted for.
We are so used to thinking about biological machines at a macroscopic level that it is all too easy to overlook the molecular structure of their individual components. The closer we inspect biochemical systems, such as flagella, the more the elegant design — as well as the magnitude of the challenge to Darwinism — becomes apparent. For further discussion of flagellar assembly, I refer readers to my review essay here. Be sure also to check out my recent response to Kelly Hughes and David Blair on the evolution of bacterial flagella and irreducible complexity.
