 Evolution
Evolution
 Intelligent Design
Intelligent Design
A One-Man Clade

The problems associated with the biological character problem [cladistics] are so complex and multifaceted and this issue is so conceptually immature that any single author’s account is doomed to be too narrow and lopsided to be of much use.
Günter Wagner1
Had Stephen Meyer better appreciated the tools of modern cladistics, Nick Matzke believes, he would not have drawn the conclusions that he did in his book Darwin’s Doubt, or argued as he had. Meyer is in this regard hardly alone. It would seem that Stephen Jay Gould was just slightly too thick to have appreciated, and the eminent paleontologist James Valentine just slightly too old to have acquired, the methods that Matzke, writing at Panda’s Thumb, is disposed to champion. Should Valentine be appointed to Matzke’s dissertation committee at UC Berkeley, we at Discovery Institute will be pleased to offer uninterrupted prayers on his behalf. We can offer no assurance of success, of course, but then again, when it comes to cladistic methods, neither can Matzke.
Why, Matzke wonders, did Stephen Meyer not include within his book cladograms such as those he himself displays in his critique, one due to Brysse, the other to Legg? He is in asking this question in full Matzke mode: Sleek with satisfaction. Meyer may well have refrained from including these cladograms because they are topologically in conflict, and display virtually no agreement with one another. Matzke’s inability to discern what is directly beneath his nose is hardly evidence of his own competence in cladistic analysis.2
It was the German entomologist Willi Hennig who with the publication of Grundzüge einer Theorie der phylogenetischen Systematik introduced biologists to his scheme of classification.3 Matzke is surely right to remark that drawing up character sets is a detailed and tedious business. But so is the work involved in alphabetizing the names of all those residents in Moscow in 1937. It is in either case no great recommendation. The great merit of cladistic analysis is just the work that it makes for cladistic analysts.4 Like so much in Darwinian biology, it is a gift that keeps on giving.
A cladistic system expresses a complicated jumble of assumptions and definitions, these expressed most often in the baroque and oddly beautiful vocabulary of Greek and Latin technical terms. No taxonomist with access to words such as paraphyletic, plesiomorphy, or synapomorphy would ever be satisfied by a description of Anomalocaris as some bug-eyed monster shrimp. Not me, for sure.Assumptions and definitions in cladistics sheathe a sturdy but simple skeleton, nothing more than a graph, lines connected to points in the plane. The blunt, no-nonsense language of graph theory is quite sufficient. A graph G = <V, E> is a collection of vertices and edges. A given vertex may be either an intersection or a terminal point of a graph. A Steiner tree is a graph spanning its terminal points. Although Steiner trees are designed to be unobtrusive, like any skeleton, they make demands all their own, most obviously because they are finite and discrete.
The cladistic classification of the living and the dead proceeds by means of a character matrix, one whose elements are bright but isolated morphological or genetic bits. Fingers are ideal. They can be counted. No organism person (typically) has more than five of them. And fingers are discrete. No one need wonder where one begins and the other ends, a point not lost on short-tempered motorists. When character sets are expressed as graphs, the result is a cladogram.
Five taxa in all: A, B, C, D, and E, individuals, species, or whole slobbering groups of them. Assume that this is so. And in 1, 2, 3, and 4, four characters. A four by five matrix suffices to display the distribution of characters, with 1 indicating that a character is present in a taxon, and 0, that it is not:
1234
A 0001
B 0011
C 1011
D 1111
E 1111
The translation of a character matrix into a cladogram can in this simple case be done by hand. Anything more complicated requires a computer program. The clade of cladists and the clade of computer programmers are on the best of terms. One clade washes the other, as professionals so often observe. Terminal points in a cladogram are occupied by the names of taxa, and vertices by their characters.
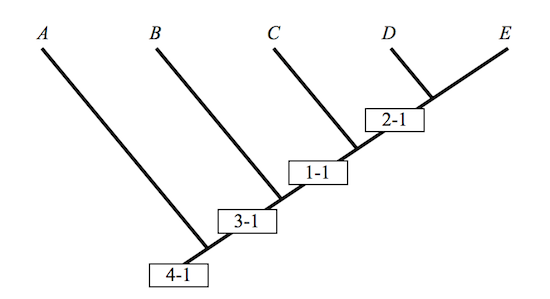
Figure 1
Aber sei vorsichtig, as Willi Hennig himself might have said, and, no doubt, would have said had he read Matzke’s critique. It is remarkably difficult to read a cladogram without reading something into it, more than the graph conveys, a bit of Darwinian doggerel most often. A, B, C, D and E are labels marking points in the plane; the taxa that they designate are found in nature. There is a difference. That A is to the left of B is a fact about graphs and labels. It makes no sense to say of two taxa that one is to the left of the other. Very few taxonomists are known widely to confuse their left and their right hands — no more than one or two. This is reassuring. That B is between A and C is otherwise. It is tempting. It is tempting precisely because it invites the taxonomist to undertake an inference from the premise that B is between A and C to the conclusion that B is somehow a descendent of A, an ancestor of C.
A cladogram does not by itself justify anything of the sort: The inference remains a non-starter because it exhibits a non-sequitur. “Evolution[ary theory] is not a necessary assumption of cladistics,” the biologist, A.V.Z. Brower once remarked.5 Neither is it sufficient, I would add. A cladogram is one way of depicting the information resident in a character matrix, and given the open-ended relationship between a matrix and its depiction in a cladogram, it is by no means unique. Intermediates that are clear as sunshine in a given cladogram disappear into darkness when the cladogram is rotated.
There is thus
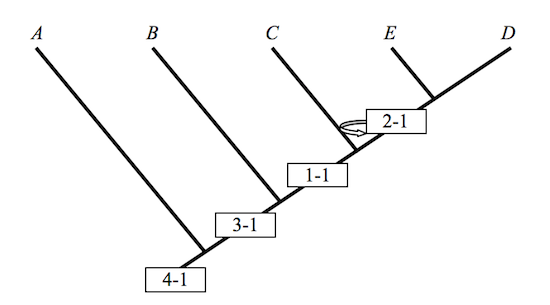
Figure 2
or even this
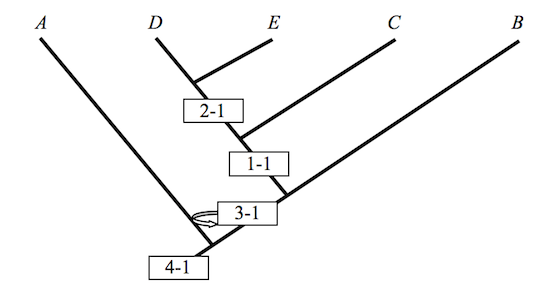
Figure 3
These cladograms preserve the hierarchical structure of Figure 1, but they fail notably to keep intermediate taxa where they once belonged. Rotations preserve some of the structure of the original, but not all of it. Similarity in structure may well be an assumption governing the identity of various cladograms; but as these examples show, structural similarity along one dimension does not preserve structural similarity along another. A cladist championing Figure 1 in his PhD dissertation is apt to see intermediates in the fossil record that his colleagues, and so his competitors, regard as nothing more than so many graph-theoretic artifacts. Never mind. Championing Figures 1 and 2 in their dissertations, they have artifacts all their own to exhibit or to hide. It is a very good thing that these people are seldom armed and rarely dangerous. If this is so, how, then, to define transitional forms? If no definition is possible, then the relevance of cladistic analysis to Darwinian biology might be more limited than often thought. It is in any case an obvious question to ask, the more so when assessing a book calling attention to the absence of transitional forms in the Cambrian era.6
Matzke acknowledges the point without grasping its meaning. “… [P]hylogenetic methods as they exist now,” he writes, “can only rigorously detect sister-group relationships, not direct ancestry, and, crucially, … this is neither a significant flaw, nor any sort of challenge to common ancestry, nor any sort of evidence against evolution.” But there can be no sisters without parents, and if cladistic analysis cannot detect their now mythical ancestors, it is hard to see what is obtained by calling them sisters. No challenge to common ancestry? Fine. But no support for common ancestry either. Questions of ancestry go beyond every cladistic system of classification, no matter the character states. It follows that questions with respect to the ancestry of various Cambrian phyla cannot be resolved by any cladistic system of classification, however its characters are defined. We are now traveling in all the old familiar circles. The claim made by Darwin’s Doubt is that with respect to the ancestors of those Cambrian phyla, there is nothing there.
The relationship between cladistics and Darwin’s theory of evolution is thus one of independent origin but convergent confusion. “Phylogenetic systematics,” the entomologist Michael Schmitt remarks, “relies on the theory of evolution.” To the extent that the theory of evolution relies on phylogenetic systematics, the disciplines resemble two biologists dropped from a great height and clutching at one another in mid-air.
Tight fit, major fail.7
No wonder that Schmidt is eager to affirm that “phylogenetics does not claim to prove or explain evolution whatsoever.”8 If this is so, a skeptic might be excused for asking what it does prove or might explain?
The graphs and trees of cladistic analysis are when examined statistically capable of recording strong signals. Matzke is right to say this.9 He is himself so alert to them that he resembles an old-fashioned commodore peering beyond mist and mizzen and hoping to see flags. While those signals may be strong, it is often unclear what they are signaling. We may imagine the world’s living cladists sorted by a complex character matrix, one involving graduate degrees, publications, tenure, citations, beard length, night sweats, beady eyes, prison records and trans-gendered identity. Their crown group comprises the living cladists together with their last common ancestor and all of his descendents. Cladistic analysis indicates, I am at liberty to disclose, that Will Hennig is the last common ancestor of all living cladists, and so an Ur-Mensch, another reason for the respect in which he is held. Having branched off early, Sokal and Sneath make for a sister group. Extinct stem groups may be seen as well, tracing their ancestry to an unter-Mensch, as German cladists say, one lost in the fog of time and more basal, if not more base, than Willi Hennig himself.
A cladogram of cladists is no different from the real thing, a cladogram by cladists. But Willi Hennig is not the last common ancestor of contemporary cladists, the gorgeous apparatus of cladistics notwithstanding, and no cladistic unter-Mensch ever existed. Common characters and common descent are not the same thing.
Cladistic methods thus suggest a number of reservations. Character matrices are the method’s heart and soul, ineliminable in practice and theory. It is precisely because character matrices are finite and discrete that cladists believe that they have on hand a body of data that they can master. “Cladistics breaks up the bodyplan characters,” as Matzke observes, “and shows the basic steps they evolved in, and also which parts of the “bodyplan” are actually shared with other phyla.”10 This is precisely what cladistics does, but what it does is at least open to the suspicion that when it comes to these issues, cladistic analysis is driven more by what cladists can do than what they should do. “No experienced naturalist,” Stephen Jay Gould remarked, “could ever fully espouse the reductionist belief that all problems of organic form might be answered by dissecting organisms into separate features …”11 By the same token, no experienced linguist would ever claim that the order in which Latin, French or German words entered the English language explains very much, if anything, about its fundamental structure.
When cladistic analysis is applied to Cambrian paleontology, the imponderables of the method reappear as obscurities in the result, an interesting example of descent with modification. In this regard, Matzke writes, “… the arthropods are instructive.” And so they are. Let us be instructed. Let us be instructed by Gregory D. Edgecombe of the Department of Paleontology at the Natural History Museum in London. “Arthropod phylogeny,” he writes cheerfully, “is sometimes presented as an almost hopeless puzzle wherein all possible competing hypotheses have support …” His conclusions hardly amount to a ringing rejection of the hopeless puzzle school. The best that he can say for his field is that it is false that “anything goes.”12 I am sure this is so.
Nick Matzke is not about to go all hopeless on his supporters either. Witness his discussion of the otherwise hideous Anomalocaris. “Anyone actually mildly familiar with modern cladistic work on arthropods and their relatives,” Matzke writes, “would realize that Anomalocaris falls many branches and many character steps below the arthropod crown group….” As it happens, Anomalocaris does not fall anywhere: It is the anomalocaridids that do the falling. They in turn are folded within Radiodonta, which makes for an order and comprises a stem-group arthropod. Their most evident character in common with the arthropod crown group is what in a lobster would be called a bony claw. It is not much, but cladists are not fussy. The anomalocaridids include the genera Anomalocaris, Peytoia, Schinderhannes, Amplectobelua and Hurdia; the arthropod stem group, the gilled lobopodians, dinocaridids, the taxon incorporating Radiodontia, fuxianhuiids and canadaspidids. It is here that characters drift between Onychophora and the arthropod crown group itself.
Without ever mentioning just which shrimp he has mind, Matzke writes that “it is one of many fossils with transitional morphology between the crown-group arthropod phylum, and the next closest living crown group, Onychophora (velvet worms).” With this remark, he solidifies his reputation as a man capable of making the same mistake twice. Common characters? Or at least one in that bony claw? Yes, of course. Transitional morphology? Ah but no. At best, an intermediate morphology. Nor can it be intermediate between the crown arthropods and Onychophora. That would be rather like placing a carrot as an intermediate between the United States and Bolivia. Wrong classification. It is Radiodonta as a taxon that is intermediate between taxa.
These are terminological disputes among us experts. A bloop is not necessarily a blunder. Let me refer in what follows to Anomalocaris X, where X designates whatever it is that Matzke had in mind. Does Anomalocaris X enter the fossil record after the first representatives of the arthropod crown make their appearance? It is in that case, a little late, one might think, to be a transitional form. Anomalocaris X could hardly be ancestral to itself nor ancestral to the trilobites and other crown group arthropods. Before then? It is in that case, a little too complex to be the ancestor of the trilobites, possessing as do all such vile bugs compound eyes more sophisticated than anything exhibited by the trilobites — more sophisticated than anything except the eyes of various dragonflies, in fact. What, then, is the ancestor of Anomalocaris X? This is just the question that Stephen Meyer asks, again and again, as it happens. It is a part of the Cambrian mystery.
It is with a question such as this that the cladistic method achieves a triumph uniquely its own. We may allow Edgecombe the last word. It is in his Figure 1 that he displays a cladogram for stem and crown group arthropoda. The figure includes Onychophora, which falls outside these stem groups but is nonetheless hoping for cladistic glory. Thick black lines move downward from various stem groups and then they stop abruptly where the evidence leaves off. The cladogram nonetheless continues recklessly down through the muck and mist of the early Cambrian, thick black lines now replaced by lines that are thin. These mark the ghost lineages of the cladist’s art, the artifacts of his method and not the imperatives of the evidence. While the last common ancestor of Radiodonta is basal to the last common ancestor of the arthropod crown, both are imaginary.
Ghost lineages are often defended, rarely extolled. Like much in cladistic analysis, they represent the withdrawal of a theory from any very robust confrontation with the evidence. They simply cannot be used to defend a view of the Cambrian that begins by questioning whether there is anything behind these ghosts beyond the cladist.
A man who believes in ghost lineages is demonstrably inclined, after all, to believe in ghosts.
References
(1) Günter Wagner, The Character Problem in Evolutionary Biology, Academic Press, 2000, preface.
(2) Depicted in Matzke, ‘Meyer’s Hopeless Monster, II,’ op cit. Brysse, Keynyn (2008). “From weird wonders to stem lineages: the second reclassification of the Burgess Shale fauna.” Studies in History and Philosophy of Science Part C: Studies in History and Philosophy of Biological and Biomedical Sciences. Legg, David A.; Sutton, Mark D.; Edgecombe, Gregory D.; Caron, Jean-Bernard (2012). “Cambrian bivalved arthropod reveals origin of arthrodization.” Proceedings of the Royal Society, Series B: Biological Sciences. 279(1748), 4699-4704.
(3) Willi Hennig, Grundzüge einer Theorie der phylogenetischen Systematik, Berlin: Deutscher Zentralverlag, 1950. No real cladist, it goes without saying, would ever refer to the English translation of this book. Let me see: I notice that Matzke never once refers to the German original. Readers must draw their own conclusions.
(4) Make-work for jerks, as a distinguished figure was heard uncharitably to remark.
(5) Brower A.V.Z. ‘Evolution is not a necessary assumption of cladistics.’ Cladistics, 2000, 16: 143-154. Later in his paper, Brower remarks that “systematics provides evidence that allows inference of a scientific theory of evolution.” What doesn’t?
(6) See Wagner G.P., Stadler P.F.. 2003. ‘Quasi-independence, homology and the unity of type: a topological theory of characters.’ Journal of Theoretical Biology 220: 505-527.
(7) A point made vividly by Matzke’s own source, which he cites in solemn incomprehension: Whatever the character matrix, Brysse observes, “… there is only enough reliable information available to construct cladograms, not trees.” Brysse, Keynyn (2008). “From weird wonders to stem lineages: the second reclassification of the Burgess Shale fauna.” Studies in History and Philosophy of Science Part C: Studies in History and Philosophy of Biological and Biomedical Sciences. 39(3), 298-313.
(8) M. Schmitt, ‘Claims and Limits of Phylogenetic Systematics.’ Z. zool. Syst. Evolu.-forsch. 27 (1989) 181 -190. Schmitt is curator of Coleoptera and Head of Department of Arthropoda at the Zoologisches Forschungsmuseum Alexander Koenig. What he does not know about beetles is apparently not worth knowing.
(9) “It is easy to calculate statistics such as CI and RI,” Matzke writes, “and compare them to CI and RI statistics calculated based on data reshuffled under a null hypothesis where any possible phylogenetic signal has been obliterated.” True enough. It is easy. “In virtually any real case, one will see substantial phylogenetic signal, even if there is uncertainty in certain portions of the tree.” True enough again. The question of what the signal is signaling remains.
(10) Matzke, op cit. By “bodyplan” Matzke means bodyplan. His remarks as written suggest someone about to dissect a word.
(11) Stephen Jay Gould, The Structure of Evolutionary Theory, Harvard University Press, 2002, p. 1057.
(12) Gregory D. Edgecombe, ‘Arthropod Phylogeny: An Overview from the Perspective of Morphology, Molecular Data, and the fossil record.’ Arthropod Structure & Development 39 (2010) 74-87. This is not a paper that lends itself to Twitter. Edgecombe is not alone. The characters Matzke thinks homologous, Legg calls into question. See the caption, Legg op. cit. Legg is Matzke’s witness and not ours. Valentine and Erwin do as much. Another witness, this one expert: ” … the lobopodians all share fairly simple, unspecialized legs, yet Opabinia and anomalocaridids lack legs but have paired, lateral flaps that, particularly in Opabinia, have gills along the upper aspect of the flap. Beyond the Radionta, however, well-sclerotized jointed appendages reappear. Are arthropod appendages homologous to those of lobopods, as Budd has argued? Are they homologous to the lateral flaps of Radionta [the group that includes anomalocaridids]? Or are they entirely novel structures? This debate is far from settled, illustrating the complexities of understanding the evolutionary pathways among these groups.” Douglas Erwin and James Valentine, The Cambrian Explosion: The Construction of Animal Biodiversity (Roberts and Company, 2013) (internal citations removed), p. 195. Vengeance is mine, saith the Lord.
Image credit: Wsytan/Flickr.

