 Intelligent Design
Intelligent Design
The Eukaryotic Cell Cycle: A Masterpiece of Design
Have you ever wondered how the eukaryotic cell knows when to transition from one phase of its cycle to the next? How does the cell know to begin DNA replication (S phase) or mitosis (M phase), or to enter the two “gap phases” (G1 and G2) that separate them? How are previous phases halted and new phases initiated? What quality-control measures are in place to minimize mistakes?
The cell cycle is under tight regulatory control, ensuring that it operates in a beautifully systematic fashion. The remarkable molecular mechanisms underlying this control constitute one of the most astonishing processes in molecular biology.
The animation above illustrates the beauty of one of the stages — indeed, the culmination — of the eukaryotic cell cycle, involving mitotic cell division. In this and a subsequent article, I will explore these processes in more detail. Before we examine the ingenious mechanisms of cell cycle control, however, I will provide here some context by means of a short description of the different phases involved. The following information regarding the cell cycle can be found in any standard biology textbook.
Introducing the Cell Cycle
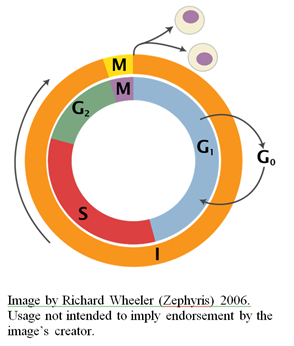
The eukaryotic cell cycle is divided into four phases, designated G1, S, G2, and M respectively (see the figure to the right). Collectively, the G1, S, and G2 phases are referred to as “interphase” (designated “I” in the figure), and take up approximately 90% of the cell’s lifespan.
The Gap Phase 1 (G1 phase) is a period of cell growth that occurs prior to chromosome duplication. During the G1 phase, cells can exit from the cycle and enter a non-dividing state called G0. Alternatively, they can pass a so-called “restriction point” which commits them to the entire cell cycle. This irreversible decision is largely dependent on nutrient availability, and often cell size (for many types of cells, commitment to another round of division requires a critical cell size). External factors known as “growth factors” also play an important role in the regulation of the transition past the restriction point. Many human cells, such as nerve cells, are permanently arrested in the G0 state. Others can be induced to re-enter the cell cycle. Still others, such as skin cells, are constantly dividing. Cells that are in the G0 state have elevated concentrations of cell cycle inhibitors and are marked by an absence of DNA replication enzymes. Cells in the G1 state, conversely, are marked by low concentrations of cell cycle inhibitors and the presence of DNA replication enzymes.
S phase is the stage at which DNA replication takes place, producing two sister chromatids (identical copies of each chromosome). These sister chromatids are tethered together by a protein called cohesion until mitosis occurs. Complex regulatory mechanisms are in place to ensure that the DNA is replicated completely and accurately before the cell is allowed to enter the next phase of the cell cycle. DNA replication is itself an absolutely remarkable process involving many different specialized protein complexes. For an overview of some of the complexities associated with DNA replication, see my previous articles on the subject here, here, here, here, here, and here.
The Gap Phase 2 (G2 phase) is a second phase of growth prior to the separation of sister chromatids. During this phase, the mitotic spindle (i.e., the apparatus responsible for driving chromosome segregation) begins to form. Cellular content also increases further at this stage (this content will later be distributed between the two daughter cells). A cell cycle checkpoint occurs at this phase, and cell cycle progression can be temporarily arrested or paused if there is any chromosome damage, such as a double-stranded DNA break, until the damage is repaired.
M phase is the final stage of the cell cycle. During this phase, two major events occur: mitosis (segregation of sister chromatids into the opposite sides of the cell), and cytokinesis (division of the cell to produce two daughter cells). Chromatids attach to the mitotic spindle (made up of microtubules that radiate from microtubule organizing centers called centrosomes) at protein structures present on the chromatids called kinetochores. The mitotic spindle pulls the sister chromatids apart, creating two identical chromosome clusters, which will go on to populate the two resultant daughter cells. As is the case for S phase and the G2 phase, there are checkpoints in place to halt cell cycle progression if there are any problems such as chromosome mis-segregation or improper attachment of chromosomes to the spindle.
The Stages of Mitosis
There are a number of different mitotic stages. These are: prophase, prometaphase, metaphase, anaphase and telophase. During prophase, the chromatin condenses into the familiar chromosome structures in which the chromatin becomes visible under the microscope. During prometaphase, the nuclear membrane disintegrates and breaks into membrane vesicles.
The kinetochores form during this stage, and become attached to the microtubules that radiate from the centrosomes at the spindle poles. During metaphase, the condensed chromosomes line up in the middle of the cell, driven by motor proteins (kinesin and dynein) associated with the microtubules. During anaphase, the chromosomes break up and the sister chromatids are pulled to the opposite poles of the cell. Finally, during telophase, which occurs at the same time as cytokinesis, two daughter nuclei are formed and the chromosomes unravel back into their original expanded chromatin formation. To better visualize the process of mitosis, I recommend viewing the video animation embedded at the top of this article. Here is another animation worth watching.
Conclusion
There is no question that the processes described above are truly astounding. In fact, when you consider the amazing regulatory control of just the microtubule-associated motor proteins (that are involved in a number of processes during mitosis, including the aligning of chromosomes along the cell’s equator during metaphase), the complexity of the system is taken to a whole new level. Later, I will discuss the even more astonishing molecular mechanisms that underpin the cell cycle’s regulation and quality control — processes that typify the sheer engineering prowess of biological machines.
