 Evolution
Evolution
 Intelligent Design
Intelligent Design
 News Media
News Media
New York Times: "We are Sarcopterygian Fish," If You Ignore Contrary Data

Given its prior history of pushing Tiktaalik as an icon of evolution (now thoroughly toppled), the New York Times has an obvious fascination with the idea that tetrapods (four-limbed vertebrates like frogs, birds, cows, and humans) evolved from fish. Now the Times Science section is at it again with an article titled “Fish’s DNA May Explain How Fins Turned to Feet.”
The article highlights a recent paper in Nature, reporting on the sequencing of the genome of the coelacanth, a famous fish often called a “living fossil” which is descended from the line of fish that supposedly evolved into tetrapods. Reporter Nicholas Wades writes that “the coelacanth is more closely related to people than to other fish,” and he quotes Axel Meyer, an evolutionary biologist who co-authored the study of the coelocanth genome, who says: “Evolutionarily speaking, we are sarcopterygian fish.” Sarcopterygians include the lungfish, coelacanth, and “evolutionarily speaking,” tetrapods like you and me.
Is Meyer right? According to the NYT, there are two main pieces of relevant evidence. First, the coelocanth has “one gene that is related to those that, in animal species, build the placenta,” and “[t]his gene could have been developed by land animals into a way of constructing the placenta.”
The real story isn’t quite that interesting. According to the Nature paper, a particular region of DNA associated with a Hox gene cluster in the coelocanth genome showed sequence homology with a stretch of Hox gene-related DNA in tetrapods. Hox genes are known to be widely conserved among vertebrates, so the fact that homology was found between Hox-gene-associated DNA across these organisms isn’t very surprising. The authors aren’t sure exactly what this particular segment of DNA does, though it’s probably a promoter region. In mice the corresponding homologous region is associated with Hox genes that are important for forming the placenta. Ergo, we’ve solved the mystery of how the placenta evolved. Right?
Not really. Again, all that was found was a little homologous promoter region in Hox-gene related DNA in these two types of organisms. Given that we don’t even understand exactly what these genes do or how they work, obviously the study offered no discussion of what mutations might have provided an evolutionary advantage. No evolutionary pathway was proposed, or even discussed. So there’s not much meat to this story, other than a nice little region of homology between two shared, functional pieces of Hox-gene-related DNA. But of course, such shared functional DNA could be the result of common design and need not indicate common descent or Darwinian evolution.
The NY Times goes on, offering the second piece of evidence: “Another helpful preadaptation is a snippet of DNA that enhances the activity of the genes that drive the formation of limbs in the embryo.” When they inserted this enhancer into mice, according to Neil Shubin, “It lit up right away and made an almost normal limb.”
What’s that he said? “Preadaptation”? Darwinian evolution isn’t supposed to have any goals, so whenever I see that term I get suspicious that there’s something non-Darwinian going on. So long as we’re discussing the coelacanth’s supposed “preadaptation” for life on the land, consider what vertebrate paleontologist Barbara Stahl wrote about coelacanth anatomy:
[T]he modern coelocanth shows no evidence of having … internal organs preadapted for use in a terrestrial environment. The outpocketing of the gut that serves as a lung in land animals is present but vestigial in Latimeria. The vein that drains its wall returns blood not to the left side of the heart as it does in all tetrapods but to the sinos venosus at the back of the heart as it does directly or indirectly in all osteicthyans except lungfishes. The heart is characteristically fish-like in showing no sign of division into left and right sides, and the gut, with its spiral-valved intestine, is of a type common to all fishes except the most advanced ray-fins.
At one time, paleontologists thought that coelocanths, like air-breathing tetrapods, had nasal passages that opened into the mouth cavity, but dissection of Latimeria disroved that idea. Despite their fleshy fins, the coelocanths were no nearer the ancestral stock of land vertebrates than the dipnoans, fishes in which internal nares, or choanae, were also shown to be nonexistent.
(Barbara J. Stahl, Vertebrate History: Problems in Evolution, p. 146 (Dover Publications, 1985).)
The coelocanth’s anatomy doesn’t make a very compelling case for “preadaptation” for land-based life, but the NY Times story about genetic “preadaptation” isn’t very compelling either. Here’s what they really found.
An enhancer is a short stretch of DNA that helps to enhance transcription of a gene by serving as an attachment point for proteins that are involved in recruiting RNA polymerase II, and various transcription factors necessary to transcribe the gene into RNA. The study found that an enhancer for a Hox gene involved in fin-development in coelocanths has sequence homology with an enhancer associated with a Hox gene involved in limb-development in mice. When the investigators inserted the coelocanth enhancer into the mouse, the mouse apparently grew what Neil Shubin called an “almost normal limb.”
Actually, the technical paper didn’t report the growth of any limbs — whether “almost normal” or totally normal — but rather only a “limb bud.” But that’s just a detail — because even if a limb had grown, this wouldn’t mean much for Darwinian evolution.
Again, it’s well known that Hox genes are conserved throughout most vertebrates, including fish (like the coelacanth) and tetrapods (like mice). In this case, the genetically homologous enhancer in the two organisms seems to have had a similar, homologous function as well: in coelacanth it enhanced a Hox gene for building fins, and in mice it enhanced a Hox gene for building limbs. This similarity of function and genetic role makes it unsurprising that that these enhancers had a similar DNA sequence. The similarity of function, genetic role, and DNA sequence is thus interesting, but it’s not overly surprising to find that it sort of worked when inserted in a mouse.
But what’s evolution got to do with any of this? The experiment worked because of functional and genetic similarities between the coelacanth enchancer and the mouse enchancer. Once again, such similarities of sequence and function could be explained by common design and don’t necessarily tell us much about common descent.
Inconvenient Immunoglobulin Data
So what are we to make of the NY Times‘s statement that “the coelacanth is more closely related to people than to other fish” or that “we are sarcopterygian fish”? Well, the article didn’t mention one important piece of evidence from the coelocanth genome that contradicts those claims.
According to the study in Nature, “The fish [coelocanth] is the first vertebrate found to lack genes for immunoglobulin-M [IgM], an almost universal immune-system protein.” That’s a bit weird, but it gets much weirder. According to the technical paper:
IgM genes cannot be found in coelacanth, despite an exhaustive search of the coelacanth sequence data, and even though all other major components of the immune system are present. Instead, we found two IgW genes; immunoglobulin genes that are found only in lungfish and cartilaginous fish and are believed to have originated in the ancestor of jawed vertebrates but subsequently lost in teleosts and tetrapods.
(Amemiya et al., “The African coelacanth genome provides insights into tetrapod evolution,” Nature, Vol. 496:311-316 (April 18, 2013) (internal citations omitted).)
This means that if we were to construct a phylogenetic tree of vertebrates based upon the IgW gene, we would get a bizarre tree that dramatically conflicts with the standard vertebrate tree. To understand why, let’s first look at the standard vertebrate phylogeny, shown in the Nature paper:
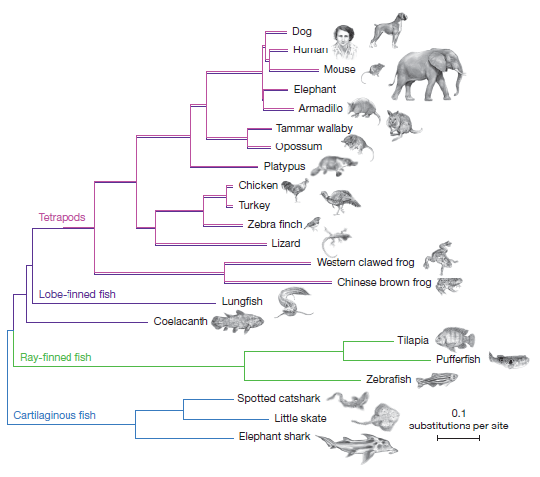
Figure A: Reprinted from Figure 1, Amemiya et al., “The African coelacanth genome provides insights into tetrapod evolution,” Nature, Vol. 496:311-316 (April 18, 2013). Image used under a Creative Commons license, Attribution-Noncommercial 2.5. Usage not intended to imply endorsement by the authors/creators of the image.
This standard phylogeny could be simplified to look something like this:
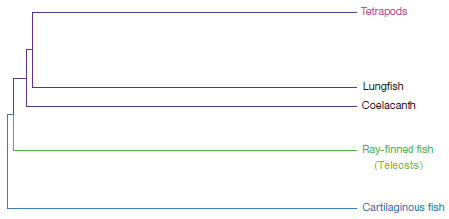
Figure B: A simplified version of the standard vertebrate phylogeny.
But according to the IgW data, very roughly speaking, the vertebrate phylogeny should look something like this:
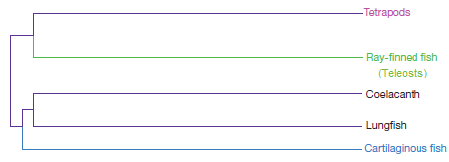
Figure C: A very rough sketch of what the vertebrate phylogeny might look like based upon the IgW gene.
Compare Figures B and C. Do you see the difference? In the standard phylogeny (Figure B), tetrapods (like you and me) are much more closely related to the coelocanth or lungfish than we are to ray-finned fish (like the goldfish). But in an IgW-based tree (Figure C), tetrapods should be much more closely related to goldfish than to the coelocanth or the lungfish. That’s startling and unexpected — if you’re a proponent of common descent.
How does the Nature paper explain this phylogenetically incongruent data? In the passage I cited above, their explanation is that “immunoglobulin genes that are found only in lungfish and cartilaginous fish and are believed to have originated in the ancestor of jawed vertebrates but subsequently lost in teleosts and tetrapods.”
Of course this requires some extremely unparsimonious and unlikely events. Since IgW is found in vertebrates as diverse as lungfish and cartilaginous fish (e.g., sharks), a Darwinian evolutionary view would infer that the gene for IgW was present in the ancestor of all jawed vertebrates. If so, then IgW must have stuck around in vertebrates long enough to end up in the sarcopterygian line. But somehow evolutionary theory must explain why tetrapods and all teleosts lack this gene.
Perhaps the loss of IgW in tetrapods isn’t very hard to explain — maybe IgW was lost in the early stages of the line that branched off other sarcopterygians and led to tetrapods.
But with teleosts, this story is much harder to believe. According to the standard evolutionary account, this ancient group of vertebrates diverged and began to diversify before the sarcopterygians (lungfish, coelocanths, and ultimately tetrapods) branched off. Since lungfish and coelocanths have IgW, somehow IgW was lost in all known teleosts, independently, and after the line that led to sarcopterygians branched off, so that today all teleosts lack this gene.
This highly unparsimonous evolutionary story is the only way (and the way the Nature paper chooses) to explain why teleosts and tetrapods lack IgW, but lungfish and coelacanths don’t. A more straightforward and plausible account of vertebrate relationships would suggest a phylogeny (Figure C) that’s quite incompatible with the standard vertebrate phylogeny.
No wonder the New York Times decided not to mention this inconvenient data point that has emerged from the coeolocanth genome: it suggests the coelacanth is NOT “more closely related to people than to other fish,” and that “we are” — yes, big surprise — NOT “sarcopterygian fish.”
Image: Natural History Museum, Vienna, Austria/Wikicommons.
