 Intelligent Design
Intelligent Design
 Life Sciences
Life Sciences
A Paper in Science Recognizes the “Design Principle” at Work in Chromosomes with Their Moving Parts
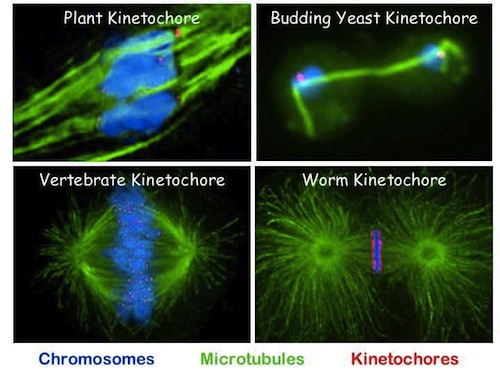
Biological jargon, until you translate it, often obscures more than it reveals. For instance, “kinetochore” simply means “moving place” in Greek. The prefix kine- (as in kinetic energy) means “move” — kinesin, therefore, is a moving thing in a cell, in this case a molecular machine that walks along microtubule highways. Our attention here is on the kinetochore: the “moving place” on the chromosome. It attaches to the centromere (central body), a piece of DNA designated as the attachment point for two “sister chromatids” (matching chromosomes). The kinetochore has the kine- prefix, because it moves during cell division, pulling its sister chromatid into the separating daughter cell.
Biology students may be familiar with the light-microscope view of cell division, wherein chromosomes are pulled apart, like bundles of worms, into the daughter cells. Those wanting to visualize this in motion will enjoy Drew Berry’s TED animation, which we recommended over the weekend, starting at 5:00 through 9:00. As cell division begins, like chromosomes pair up along centromeres (jargon for “central bodies”) with kinetochores as attachment points for the microtubules that will pull them apart. As Berry says, kinetochores contain some 200 different kinds of proteins, thousands of proteins in all. Collectively, kinetochores look like thick, round docking stations on opposite sides of the chromosome, where long strands of microtubules attach.
Through a light microscope, students and scientists have long noticed the paired chromosomes oscillating back and forth before they split apart. Those movements don’t just happen; there are machines (dynein and kinesin) pulling them toward the pole (P) and away from the pole (AP) to jostle them into place along the central axis until the signal is given to divide. Upon that signal, the spindle poles winch the separated chromosomes into the daughter cells by controlling the assembly and disassembly of microtubule building blocks.
Now that molecular biologists have become increasingly familiar with cellular machinery at the nanometer level, they realize that such movements must obey the laws of physics: laws involving force, friction, plastic deformation, spring action and more. A July 20 paper in Science explored how “Deformations within Moving Kinetochores Reveal Different Sites of Active and Passive Force Generation.” From the outset, the research team considered these systems as mechanical devices subject to physical forces:
Active interfaces generate pulling force by transducing the free energy of microtubule plus-end depolymerization into mechanical work at kinetochores moving poleward (P). Passive interfaces generate molecular friction when kinetochores are forced to slide over the microtubule lattice toward plus-ends. This occurs when kinetochores moving away from poles (AP) are pulled by P sisters and also when poleward flux pulls microtubules away from stationary kinetochores in some systems.
Mechanical compliance (deformation in response to force) should report on the position and direction of forces acting on kinetochores. We used the separation between red and green probes to measure kinetochore deformation in living Ptk2 cells.
In their research, Dumont, Salmon and Mitchison placed glowing markers inside the kinetochore of a chromosome, attached to some of the known proteins. In this way, they were able to specify regions of friction and force generation as well as measure some of the forces involved. They identified the P kinetochore being pulled against friction by an active force-generating mechanism, something like a spring being compressed. On the opposite side of the chromosome, the AP kinetochore experienced passive force generation, like a spring being stretched while attached to a fixed object (in this case, opposite spindle pole). Both the AP and P kinetochores use the passive element to generate force in one direction (for the sake of illustration, let’s say, to the right), whereas the P kinetochore has an active element closer to the chromosome pulling left — compressing the “spring” in the space between the elements. Thus they determined that there were mechanical, not biochemical processes at work.
The authors did not mention evolution (in the sense of how the system came to be), but in the climactic last paragraph, they used the phrase “design principle”:
Spatially separated passive and active interfaces at kinetochores, whether comprising different molecules or different interactions of the same molecule with microtubules in different locations, may represent a design principle with important advantages. The passive frictional interface binds persistently to microtubules independently of the microtubule dynamics state or movement direction, ensuring segregation accuracy. The active interface consumes energy to efficiently move kinetochores poleward but can evolve without the constraint of requiring persistent attachment. Together, both interfaces allow the kinetochore to harness force from depolymerizing microtubules without losing grip. That said, kinetochores may be able to function using only the passive interface — for example, in systems without anaphase A or where microtubules polymerize continuously at kinetochores, even during anaphase. In these systems, segregation forces will be generated elsewhere in the spindle and presumably transmitted to chromatin via molecular friction.
What this implies is that the “design principle” is not absolutely essential for survival (since some organisms, like roundworms, get by without the active force generating mechanism), but that it supplies “important advantages” to those having it. Needless to say, any “design principle” or non-essential advantage is hard to explain by Darwinian mechanisms, to say nothing of the mind-boggling complexity in Drew Berry’s animation. Kinetochores, as Berry explains, have been under intense study for over 100 years, and “we’re still just beginning to discover what it’s all about.” Like the authors of the Science paper, he also did not offer an evolutionary explanation for these structures with their “signal broadcasting system” and all.
By contrast, intelligent design is intuitively obvious to the open mind. As Jonathan Wells remarked near the end of Unlocking the Mystery of Life, after an hour of evidence, “when I look at the evidence objectively, without ruling out the possibility of design, design just ‘leaps up’ as the most likely explanation; and that’s why I believe that it’s true.” Machines with moving parts that obey the laws of physics while performing goal-oriented functions — our uniform experience with such systems insistently proposes that the best explanation is intelligent design.
Image credit: University of Illinois at Urbana-Champaign.
