 Evolution
Evolution
 Life Sciences
Life Sciences
Nature‘s “Gems”: Microevolution Meets Microevolution
| Links to our 9-Part Series Responding to Nature‘s Evolution Evangelism Packet:
• Part 1: Evaluating Nature’s 2009 “15 Evolutionary Gems” Darwin-Evangelism Kit |
In Nature‘s evolution-evangelism packet, one of the “evolutionary gems” on offer was titled “Microevolution meets macroevolution,” implying that it would show how small-scale evolutionary change could lead to large-scale changes. The evolution-evangelism packet explains, observing a difference between “microevolution” and “macroevolution”:
Darwin conceived of evolutionary change as happening in infinitesimally small steps. He called these “insensible gradations,” which, if extrapolated over long periods of time, would result in wholesale changes of form and function. There is a mountain of evidence for such small changes, called microevolution — the evolution of drug resistance, for instance, is just one of many documented examples.
We can infer from the fossil record that larger species-to-species changes, or macroevolution, also occur, but they are naturally harder to observe in action. That said, the mechanisms of macroevolution can be seen in the here-and-now, in the architecture of genes. Sometimes genes involved in the day-to-day lives of organisms are connected to, or are even the same as, those that govern major features of animal shape and development. So everyday evolution can have large effects.
With high hopes finding a genetic change that shows “large effects” on an organism, the reader is then directed to a paper co-authored by Sean Carroll. The paper discussed changes in fruit flies thought to result from mutations in cis-regulatory elements of a gene controlling wing pigmentation. And what was the “large effect” that shows “macroevolution”? The article states:
Here we identify one of the molecular mechanisms that contributes to the evolutionary gain of a male-specific wing pigmentation spot in Drosophila biarmipes, a species closely related to Drosophila melanogaster. We show that the evolution of this spot involved modifications of an ancestral cis-regulatory element of the yellow pigmentation gene.
(Nicolas Gompel, Benjamin Prud’homme, Patricia J. Wittkopp, Victoria A. Kassner & Sean B. Carroll, “Chance caught on the wing: cis-regulatory evolution and the origin of pigment patterns in Drosophila,” Nature, Vol. 433:481-487 (February 3, 2005).)
That’s right: All this hype about “large” evolutionary effects and “microevolution meets macroevolution,” yet all they’ve attempted to explain is the origin of small-scale coloration changes in spots on fruit fly wings. If you want to understand the underwhelming nature of these spots, just look at the dark spot on the end of the wing of Drosophila biarmipes in the diagram below:
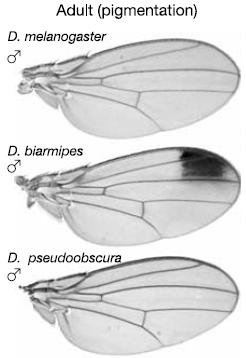
Adapted by permission from Macmillan Publishers Ltd: Nature, Figure 1, Nicolas Gompel, Benjamin Prud’homme, Patricia J. Wittkopp, Victoria A. Kassner & Sean B. Carroll, “Chance caught on the wing: cis-regulatory evolution and the origin of pigment patterns in Drosophila,” Nature, Vol. 433:481-487 (February 3, 2005). Copyright 2005.
As I remarked previously with regards to small-scale coloration changes in guppies, again bear in mind that it is minor coloration variations like these which Nature claimed would show “just what is the evidence for evolution by natural selection.” Does this show “macroevolution”?
Apart from the obvious fact that this paper has not shown that changes in cis-regulatory regions of genes are capable of explaining large-scale evolutionary change, it’s worth noting that some leading evolutionary biologists have been highly critical of those who seek to explain evolution by appealing to such regulatory mutations.
Two years after this Nature gem was published, Hopi E. Hoekstra and Jerry Coyne wrote a review article in the journal Evolution offering a potent rebuttal to arguments from “evo-devo” based upon changes in cis-regulatory elements:
An important tenet of evolutionary developmental biology (“evo devo”) is that adaptive mutations affecting morphology are more likely to occur in the cis-regulatory regions than in the protein-coding regions of genes. This argument rests on two claims: (1) the modular nature of cis-regulatory elements largely frees them from deleterious pleiotropic effects, and (2) a growing body of empirical evidence appears to support the predominant role of gene regulatory change in adaptation, especially morphological adaptation. Here we discuss and critique these assertions. We first show that there is no theoretical or empirical basis for the evo devo contention that adaptations involving morphology evolve by genetic mechanisms different from those involving physiology and other traits. … Genomic studies lend little support to the cis-regulatory theory…
(Hopi E. Hoekstra and Jerry A. Coyne, “The Locus of Evolution: Evo Devo and the Genetics of Adaptation,” Evolution, Vol. 61-5: 995-1016 (2007).)
Significantly, the authors argue that “evo devo’s enthusiasm for cis-regulatory changes is unfounded and premature. There is no evidence at present that cis-regulatory changes play a major role — much less a pre-eminent one — in adaptive evolution.” In fact, Hoekstra and Coyne argue that in this very “gem” cited by Nature, the evidence linking phenotypic change to cis-regulatory elements has not been fully established:
Although in this case the promoter region clearly contains regulatory modules controlling the spatial expression of yellow, the direct link between genotype and phenotype is not complete. This is because changes the cis-regulatory elements of yellow alone are not sufficient to produce the phenotype of interest — the pigmented wing spot (Gompel et al. 2005). Additional loci must therefore be involved. Although it is not surprising that different cis-regulatory elements in the yellow promoter affect yellow expression, a critical piece of evidence is still missing: the demonstration that species-specific cis-regulatory elements produce the species-specific difference in the wing spot.
(Hopi E. Hoekstra and Jerry A. Coyne, “The Locus of Evolution: Evo Devo and the Genetics of Adaptation,” Evolution 61-5: 995-1016 (2007).)
Given that Nature‘s evolutionary gem admits “the shape of the wings and the pattern of venation have not changed much over 60-80 Myr of evolution,” perhaps Nature‘s efforts would be better spent trying to explain why species don’t change rather than making stretched arguments that small changes in the color of wings are “large effects” that actually do show how “microevolution meets macroevolution.”
In fact, the 15th gem in Nature‘s packet tries to explain “Variation versus stability” and account for the fact that “Species can remain mostly unchanged for millions of years, long enough for us to pick up their traces in the fossil record. But they change, too, and often very suddenly.” The implication, of course, is that the evolutionary change does not last “long enough for us to pick up their traces in the fossil record” — i.e., we don’t find transitional fossils. Can this “gem” cited by Nature explain how rapid evolutionary change takes place?
According to the paper Nature cites (Aviv Bergman & Mark L. Siegal, “Evolutionary capacitance as a general feature of complex gene networks,” Nature, Vol. 424:549-552 (July 31, 2003)), some genes serve as “evolutionary capacitors” which inhibit “variation under normal conditions, thereby promoting the accumulation of hidden polymorphism.” When these genes are “knocked-out,” the authors speculate this allows hidden genetic variation to lead to phenotypic change. They spell out exactly what they are hypothesizing: “most, and perhaps all, genes reveal phenotypic variation when functionally compromised” and “the availability of loss-of-function mutations accelerates adaptation to a new optimum phenotype.”
The aforementioned article by Hoekstra and Coyne might be instructive here. They argued that the case for evolution by changes in cis-regulatory elements is weak because “Supporting the evo devo claim that cis-regulatory changes are responsible for morphological innovations requires showing that promoters are important in the evolution of new traits, not just the losses of old ones.” This criticism seems applicable to the final Nature gem as well: If phenotypic evolution occurs during “loss-of-function mutations” where genes are “functionally compromised” or “knocked out,” then how do we explain the evolution of new genes in the first place? At some point evolution cannot proceed simply by “loss-of-function mutations.”
Nature‘s evolution-evangelism packet isn’t forthcoming about the fact that this paper has made the ultimately impotent proposal that evolution proceeds by “loss-of-function mutations.” The packet claims that Bergman and Siegal “showed that most, and perhaps all, genes hold variation in reserve that is released only when they are functionally compromised. In other words, it looks as if evolutionary capacitance might go wider and deeper than Hsp90.” But the paper does not support these grand claims.
First, it admits that the gene that was knocked out — Hsp90 — might be a severe case since it serves as a chaperone protein, and most other potential evolutionary capacitors “might have more subtle effects than Hsp90 has.”
Second, Bergman and Siegal’s study used a computer simulation to “ask how an arbitrary null (‘knockout’) mutation affects the expression of other genes.” But without using real experiments knocking out real genes in real organisms, they have not actually tested whether knocking out genes might have deadly or deleterious effects. They recognize this is important, writing, “At this point one might ask whether there is any corroborating experimental evidence that knockout mutations tend to increase variation in the expression of other genes.”
The paper answers “there is,” but the authors don’t tell the reader about the numerous deleterious effects that occurred in experiments that knocked out Hsp90 in fruit flies. Likewise, Nature‘s evolution-evangelism packet says that when Hsp90 is compromised, “the proteins it normally regulates are left to run free, producing a welter of otherwise hidden variation.” However, the original research found that various “developmental defects” occurred in fruit flies where Hsp90 was knocked out, including deformed eyes, deformed legs, malformed tergites, and various other abnormalities that would never lead to viable variation in the wild. (See Suzanne L. Rutherford & Susan Lindquist, “Hsp90 as a capacitor for morphological evolution,” Nature 396:336-342 (November 26, 1998).) If such deleterious deformities are the types of “variation” observed, then it would seem that this mechanism is not going to foster viable evolutionary novelty in the wild.
In fact, a commentary on this “gem” noted that “the authors looked only at gene expression (the ‘transcriptome’), rather than at anything approaching the complexity of whole-organism phenotype” — so the all-important question of what organism-wide effects would be produced by knocking out these genes has not been investigated. As the critique explains, the effects of knocking out these genes might be deleterious:
[V]ariation released in the transcriptome could also produce changes in reproduction and mortality rates, which determine the strength of natural selection. If increased genetic variation were accompanied by reduced variation in reproductive success, or by reductions in the correlation between variation in traits and variation in reproductive success, the rate of evolution would not necessarily change. In fact, if the effects were strong enough, the rate of evolution might even decrease.
(Stephen C. Stearns, “Safeguards and spurs,” Nature 424:501-504 (July 31, 2003).)
While the study by Bergman and Siegal might show that gene expression changes when a gene is knocked out, this doesn’t account for the effects on the organism stemming from (1) the loss of a gene, and (2) differential expression of other genes. To repeat the basic question here: “Does evolutionary capacitance explain the periods of stasis and rapid change that are seen in experimental studies of evolution — or perhaps even in the fossil record?,” Stearns’s critique of this paper makes it clear that the question has not yet been answered in the affirmative. Perhaps, the deleterious effects observed in organisms that have lost such “capacitor” genes suggest that the question has indeed been answered — and that the answer is no.
