 Evolution
Evolution
 Life Sciences
Life Sciences
Understanding Ontogenetic Depth, Part II: Natural Selection Is a Harsh Mistress
Longer post today. Bear with me. [For a little background, be sure to read Understanding Ontogenetic Depth, Part I.]
1. What Does “Traversing Ontogenetic Depth” Mean?
Let’s look again at Toy Organism Alpha. Figure 1 depicts a complete cell lineage — “complete” in the sense that the starting point is a single cell, and the end point, a reproductively capable adult. Adults, by definition, can produce the specialized cells (gametes), which, when fertilized, start the whole process of cell division and differentiation again.
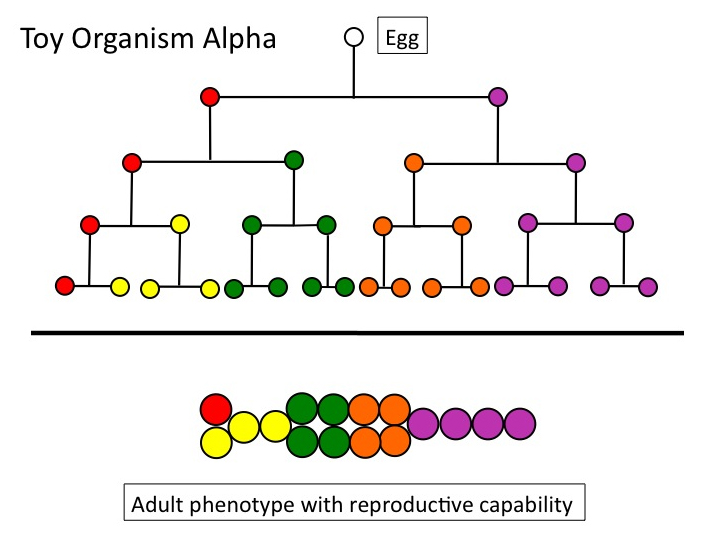
Figure 1
Once upon an evolutionary time, this lineage did not exist. (Alpha is only an illustration, of course; I mean cell lineages such as are found in real animals, which are vastly more complicated.) It had to be constructed, incrementally, by some undirected process. Under textbook neo-Darwinian theory, that process was descent with modification via the natural selection of randomly-arising variation. I’ll refer to this theory by the shorthand “natural selection.” Traversing ontogenetic depth means simply that any candidate evolutionary process (natural selection, genetic drift, self-organization, whatever) must build across — “traverse” — a distance of increasing complexity, from an aboriginal single-celled state, to multicellularity, to a genuine dividing and differentiating lineage. That distance cannot be crossed in a single increment of change.
Now, before we consider what natural selection requires, let’s strip down Alpha’s cell lineage to just the first two rounds of cell division, yielding four cell types:
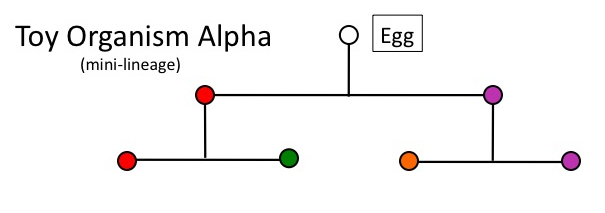
Figure 2
This will provide our model for the problem of traversing ontogenetic depth via natural selection. Recall that the process of natural selection (Endler 1986) has three jointly necessary and sufficient conditions, which may be expressed by the following conditional:
If, within a species or population, the individuals
a. vary in some attribute or trait q — the conditions of variation;
b. leave different numbers of offspring in consistent relation to the presence or absence of trait q — the condition of selection differences;
c. transmit the trait q faithfully between parents and offspring — the condition of heredity;
then the frequency of trait q will differ predictably between the population of all parents and the population of all offspring.
Could this process have constructed the mini-lineage in Figure 2? Let’s see. We’re going to try to traverse ontogenetic depth, step-by-step, via an undirected process. We can set aside for the moment condition (b), selective differences, and focus just on conditions (a) and (c), variation and heredity.
The starting point will be a single cell — call it Cell Zero — which divides:
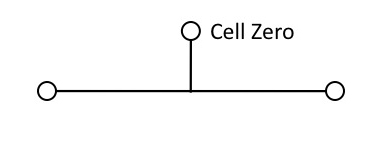
Figure 3
But that won’t work for building the lineage, unless the daughter cells remain physically connected. So Cell Zero needs the instruction, (1), “divide and stick together.” Obviously, this instruction must be in place before Cell Zero divides.
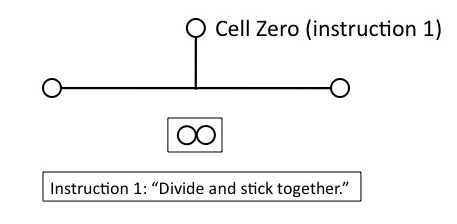
Figure 4
Now we have two daughter cells, but they’re identical. Unless we provide another instruction to Cell Zero, however, we’re going to produce a clonal mass of identical cells: no differentiation will occur, which we need to construct the lineage. Cell Zero therefore needs another instruction, (2), “daughter cells differ,” which again must be in place before Zero divides.
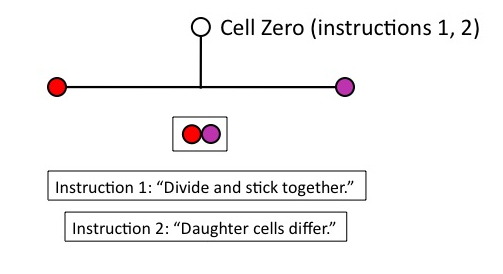
Figure 5
Then the two daughters divide, yielding four cells of four different types. Have we successfully constructed the lineage via natural selection?
No; to this point, natural selection has caused nothing, because one of its necessary conditions, heredity, has not been satisfied. This organism (of four differentiated cells) must leave progeny, to which it transmits its distinctive variations. Condition (c) of the process of natural selection, heredity, requires that variations arising in a parent be transmitted to offspring. Heredity thus entails that at least one of four cells keep track of the instruction set for the lineage as a whole, if the cycle of differentiation is going to repeat (i.e., be maintained) in the next generation.
Cell Zero therefore needs yet another instruction, (3), in place before it divides: “One cell line must keep track of the whole instruction set, and become the starting point for another cycle of differentiation.” This is the functional reason animals need something like a germ line (i.e., cells which produce gametes): those cells will be the instruction-minders, or instruction-carriers, for the organism as a whole.
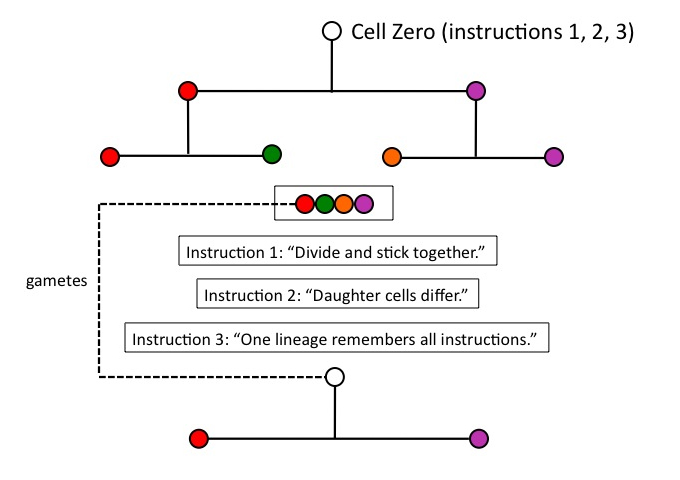
Figure 6
Is natural selection operating yet? Nope: thus far, we’re building an egg, namely, Cell Zero, by loading it with the functionally necessary instructions that must be in place before it divides. Reproduction of the whole lineage, which will enable selective differences (condition b) to arise, is causally well downstream. Whatever instructions must be present in Cell Zero, therefore, have to be there BEFORE natural selection can operate.
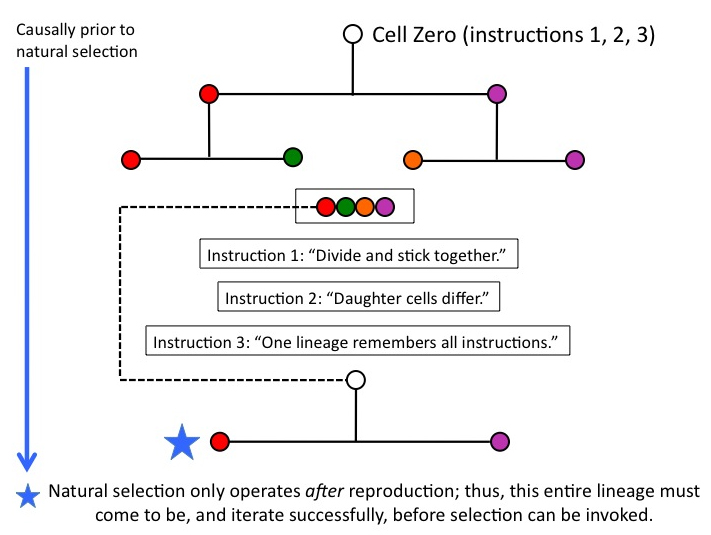
Figure 7
We’ve just traversed a relatively short interval of ontogenetic depth (OD). And the process of natural selection, harsh mistress that she is, stands entirely to one side, indifferently watching us do the work of building an egg. Not until we have produced a lineage capable of replicating itself with fidelity can natural selection begin to operate.
2. Try This Yourself, With a Pencil and a Sheet of Paper: P.Z. Myers Already Gave Me His Solution, Which Doesn’t Work
There’s nothing especially biological about all this. Figures 3 through 7 depict a decision tree, and what would be required, as instructions in the starting node (Cell Zero) to specify that tree and cycle it through successive generations. You can build such trees yourself, to any degree of complexity your patience will tolerate. Try to find the minimal set of instructions required in the starting node to generate the differentiated end-nodes, and to repeat the process from any one of those end-nodes. Keep in mind that, if you want the whole lineage to repeat (“reproduce”) with fidelity, any decision affecting any end-node must be front-loaded into the starting node.
In late July 2004, at the Society for Developmental Biology annual meeting, held at the University of Calgary, P.Z. Myers and I talked about this very problem, at length. Myers stopped by my poster (which he pronounced wretched, by the way) and we found ourselves discussing what the “Urbilaterian” — the putative ancestor of the bilaterally symmetrical animal phyla — might have resembled. (In my poster, I argued that no one had successfully described Urbilateria because it is impossible to do, an argument I endorse even more strongly today.) Since Urbilateria, if it existed, would have been a metazoan and undergone a developmental process of its own, Myers and I wrangled about how that process would have evolved de novo — just the problem we’ve been sketching above.
Myers told me I had greatly exaggerated what was a boringly simple puzzle. To illustrate his point, he drew me a picture (which I still have somewhere, I think), that looked like this:
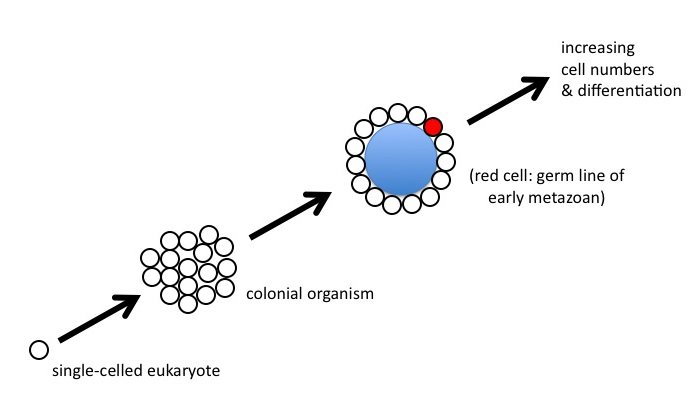
Figure 8
“It’s no real problem, Paul,” Myers said. “Start with a eukaryote that becomes colonial. The cells of the colony start specializing, maybe a central cavity forms, and then some of the cells repress their gene expression and become a kind of germ line for the rest of the colony, which is evolving into a true animal. No big deal.”
I have to admit when Myers told this story, he expressed it with such aplomb that, at the time, I could only smile. But as I contemplated his drawing, I realized what he had sketched could not possibly work in any realistic evolutionary scenario.
Indeed, it then struck me that I had seen Myers’ scenario many times before. Evolutionary developmental biologist Lewis Wolpert — whom no one, even in his wildest delirium, would ever mistake for an ID theorist — had long critiqued the scenario on functional grounds, using what he called “the continuity principle.” (1994) The continuity principle requires that any change occurring in an evolutionary transformation be biologically possible, that is, viable and stably heritable in the next generation.
“Since embryonic development requires the formation of a multicellular organism from a single cell,” Wolpert observes (1994, 79), “the origin of the egg is a central and sadly neglected problem.” The functional challenge to be solved is the origin of heritable differentiation:
The key to all development is the generation of differences between the cells, that is, making them non-equivalent [see Figures 1 and 2, above]. Only if the cells are different can the organism be patterned so that there are organized changes in shape, and cells at specific sites differentiate into different cell types. How could this have evolved? (Wolpert 1994, 80)
Look again at Figure 8, and think about what would need to happen between the “colonial” stage and the origin of the germ line (the red cell at the third stage). What mechanism is coordinating gene expression among all the members of the colony, such that only one cell lineage will evolve to carry the complete instruction set required to specify the form of the whole? How are mutations — occurring in all individual cells of the colony — transmitted to the next generation? If individual cells continue to reproduce via normal fission, or budding, notes Wolpert, “cell lineages [will be] mutating in all sorts of directions in genetic space.” (2002, 745) Given such genetic chaos, he argues, “we consider it practically impossible” for the collection of cells to “yet retain the ability to evolve into viable new forms.”
To modify irreversibly the global form of any animal, Wolpert contends, requires mutations affecting a single key cell — the egg. Mutations affecting somatic cells — especially when every cell, during the transition from colonial organism to true metazoan, is possibly either “somatic” or “germ line” — cannot be coordinated:
There is no way that the genes in the huge number of cells…can change at the same time, and mutations in individual cells mean that they no longer share the behavioural rules of the majority. It is only through a coherent developmental programme, with all cells possessing the same genes, that organisms can evolve, and this requires an egg. (2002, 745)
At the moment, the problem of the evolutionary origin of cellular differentiation stands open, for the reasons outlined above. “How cell types of multicellular organisms came to be differentiated,” noted Carl Schlichting (2003, 98), “is still an open issue.” The problem, I think, cannot be solved within a neo-Darwinian (natural selection) framework, given that selection as a causal process cannot fix variants whose selective differences (condition b of selection) lie in the future. Building de novo the distantly end-directed pathways that characterize animal development, however, seems to require fixing just such variants.
To recapitulate my thesis, then:
The theory of evolution by natural selection does not explain the origin of animal form, because natural selection cannot account for origin de novo of the developmental stages required to construct (i.e., evolve) animals. The concept of ontogenetic depth helps us to understand why.
There’s a lot more to say, but this outlet doesn’t favor the long form. Let me end, however, with one final point, which I’m hoping P.Z. Myers will address.
3. How Does Early Development in Animals Evolve?
It is unclear how ontogenetic architectures early in metazoan history could have differed fundamentally from present-day systems. The problem may be summarized as follows:
— There are striking differences in the early development in animals, even within classes and orders.
— Assuming that these animals are descended from a common ancestor, these divergences suggest that early development evolves relatively easily.
— Evolution by natural selection requires heritable variation.
— But heritable variations in early development, in major features such as cleavage patterns, are not observed.
Extant species provide many case studies in the puzzle of modifying early development. Remarkable examples of early ontogenetic divergence abound (e.g., in nematodes; Felix 1999; Schierenberg 2001). These divergences have now become a commonplace of the evo-devo literature, and are taken as prima facie evidence that early development evolves dramatically. “It is clear that a casualty of [these comparative data],” argues Davidson (1990, 384), “is the 19th century concept that early development must be an evolutionarily conserved process” — a concept, interestingly enough, which Davidson himself advocated in 1971: “One can imagine modest alterations or additions to the early parts of the developmental program,” he wrote at the time, “but it would be very unlikely that such programs could be supplanted.” (1971, 131)
There exists a striking paucity of experimental evidence showing heritable variation in what Wimsatt and Schank (1988) call the “deeply-entrenched” features of metazoan development. Why is such deep variation so hard to find? Again, we should ask, what does the logic of natural selection require?
A. Variation in a trait q
B. Fitness differences in consistent relation to the presence of trait q, and
C. Heritability of trait q.
The experimental literature on model systems such as Drosophila describes many mutations in early developmental characters and patterns. With rare exceptions, however, such mutations are not heritable, in the sense that the phenotypes exhibited do not survive as stably-breeding lines. As a result, some have argued that we should not expect mutagenesis to reveal the basis of adaptive variation: “The take home message,” argues Nagy (1998, 820), “is that mutagenesis in model systems does not undo evolution or reveal, in any direct fashion, the basis of evolutionary change.”
But if the experimental literature does not provide evidence of heritable deep variation, how do we know that such changes are even possible? “Comparative embryology abounds,” argue Wray and McClay (1989, 811), “with empirical evidence of evolutionary modification of early development.” The theory of common descent, of course, underwrites the assumption. Because the animals are believed to have descended from a common ancestor, therefore it must be possible for early development to vary heritably. “So the dilemma is easily solved,” argues Thomson (1992, 112). “Because early stages have changed, they must be capable of change.”
But what if the evidence from model systems continues to suggest that fundamental variation in early ontogeny is not heritable?
References
Arthur, Wallace. 2004. Biased Embyos and Evolution. Cambridge: Cambridge University Press.
Britten, R. and Davidson, E. 1971. Repetitive and non-repetitive DNA sequences and a speculation on the origins of evolutionary novelty. Quarterly Review of Biology 46:111-38.
Davidson, Eric. 1990. How embryos work: a comparative view of diverse modes of cell fate specification. Development 108:365 389.
Davidson, Eric and Erwin, Douglas. 2010. An Integrated View of Precambrian Eumetazoan Evolution. Cold Spring Harb Symp Quant Biol 74:1-17.
Dawkins, Richard. 1982. Replicators and Vehicles. In Current Problems in Sociobiology, eds. Kings College Sociobiology Group. Cambridge: Cambridge University Press.
Endler, John. 1986. Natural Selection in the Wild. Princeton: Princeton University Press.
Felix, Anne-Marie. 1999. Evolution of Developmental Mechanisms in Nematodes. Journal of Experimental Zoology (Mol Dev Evol) 285:3-18.
Miklos, G.L.G. 1993. Emergence of organizational complexities during metazoan evolution: perspectives from molecular biology, palaeontology and neo-Darwinism. Mem. Ass. Australas. Palaeontols 15:7-41.
Nagy, Liza. 1998. Changing Patterns of Gene Regulation in the Evolution of Arthropod Morphology. American Zoologist 38:818-828.
Nelson, P. and Ross, M. 2003. Understanding the Cambrian Explosion by Estimating Ontogenetic Depth. [abstract] Developmental Biology 259:459-60.
Schierenberg, Einhard. 2001. Three sons of fortune: early embryogenesis, evolution and ecology of nematodes. BioEssays 23:841-847.
Schlichting, C.D. 2003. Origins of differentiation via phenotypic plasticity. Evolution & Development 5:98-105.
Thomson, Keith S. 1992. Macroevolution: The Morphological Problem. American Zoologist 32:106-112.
Wimsatt, William and Schank, Jeffery. 1988. Two constraints on the evolution of complex adaptations and the means for their avoidance. Pp. 231-273. In Nitecki, M.H. (ed.) Evolutionary Progress. Chicago: University of Chicago Press.
Wolpert, L. 1994. The evolutionary origin of development: cycles, patterning, privilege and continuity. Development [Supplement] 70:79-84.
Wolpert, L and Szathm�ry, E. 2002. Evolution and the egg. Nature 420:745.
Wray, Gregory and McClay, David R. 1989. Molecular Heterochronies and Heterotopies in Early Echinoid Development. Evolution 43(4):803 813.
